Abstract
BACKGROUND:
Chronic obstructive pulmonary disease (COPD) exacerbations can accelerate disease progression and lead to higher health care costs. To improve patient survival and reduce cost, risk assessment measures should be developed to identify patients at risk for exacerbations and prevent future exacerbations.
OBJECTIVES:
To (a) externally validate the COPD treatment ratio (CTR) as a measure of COPD exacerbation risk based on predictive models previously tested and (b) assess the measure’s capability to assess risk using only pharmacy claims for use in Medicare Part D programs.
METHODS:
This was a retrospective observational study conducted using the Humana research datasets. Separate assessments were performed using pharmacy-only models that excluded risk factors derived from medical claims. Patients were aged ≥ 40 years, with ≥ 1 inpatient hospitalization or ≥ 2 physician’s office, emergency department, or urgent care visits with a COPD diagnosis. Using logistic regression models, risk factors (age, exacerbation history, COPD and concomitant medication use, and comorbidities) were assessed during the baseline period (year 1) and were used to predict the risk of exacerbation during year 2. Continuous and dichotomized CTRs were analyzed. A cut-point of 0.3 was initially used for dichotomizing CTR, and subsequently receiver operating characteristics (ROC) analysis was used to determine the optimal cut-point for CTR.
RESULTS:
A total of 92,496 patients were identified, the majority of which (96.2%) were Medicare members with a mean age of 69 years. During the baseline period, 14.0% and 11.2% of patients had ≥ 1 moderate or severe exacerbation, respectively. Overall, the CTR performed well in predicting future COPD exacerbations, especially severe exacerbations. ROC analysis suggested that 0.7 was the optimal cut-point for dichotomizing CTR. Patients with a CTR ≥ 0.7 had a 7.9% (OR = 0.921; 95% CI = 0.852-0.995) lower risk of a severe exacerbation, compared with those with a CTR < 0.7. Stronger effects were seen in pharmacy-only models, with patients 17% less likely to experience a severe exacerbation with a CTR ≥ 0.7 compared with patients with a CTR < 0.7.
CONCLUSIONS:
This study validated the use of CTR as a modifiable measure of risk of COPD exacerbation in a large commercial and Medicare population and remained a robust predictor when pharmacy-only claims data were available. A CTR of ≥ 0.7 may be a useful target to help reduce the risk of severe exacerbations, and its use by payer or quality organizations has the potential to improve COPD management.
What is already known about this subject
Chronic obstructive pulmonary disease (COPD) exacerbations have a long-term detrimental effect on patients and are associated with higher health care costs.
There is significant interest in strategies to assess COPD exacerbation risk and to help patients avoid exacerbations, but current tools rely on medical records that are not readily available.
The COPD treatment ratio (CTR) was developed and validated in a consolidated managed care database and further validated in an integrated delivery network.
What this study adds
This study further validated the use of CTR to predict future exacerbations using data from the Humana commercial and Medicare database.
CTR reliably assessed exacerbation risk using pharmacy-only claims data.
Chronic obstructive pulmonary disease (COPD) is a progressive disease characterized by persistent airflow limitation and respiratory symptoms, including chronic and progressive dyspnea, cough, and sputum production, and is often complicated by exacerbations.1 COPD exacerbations (periods of acute worsening of respiratory symptoms) are of major importance in terms of their prolonged detrimental effects on patients, acceleration of disease progression, and high health care costs.1,2 Services related to exacerbations comprise a substantial portion of the total cost of managing COPD,2,3 which was estimated at $32.1 billion in 2010.4 More frequent and severe exacerbations are associated with higher rates of health care resource utilization and associated costs.5 The potential to avoid COPD exacerbations has fueled interest in strategies to reduce the number of exacerbations, which will not only improve patient outcomes but reduce costs. Hence, risk assessment tools that can identify patients at greatest risk for future exacerbations can help with targeting patients to help reduce exacerbations over time.
While previous history of exacerbations is the most important predictor of future exacerbations,6 additional risk factors have been identified and used to develop exacerbation risk models.7-10 Health insurance claims represent a readily available source of data to inform health plans and quality of care organizations, which may be used to identify patients at high risk of exacerbations.11 The COPD treatment ratio (CTR) model has previously been validated as a reliable predictor of moderate or severe exacerbation risk using commercial-only claims data from MarketScan, a large employer-based managed care claims database, and Reliant Medical Group, an integrated delivery network.11,12 Implementing such a simple measure and reporting it systematically to providers gives them an easily interpretable assessment of exacerbation risk from which additional targeted interventions may be devised to reduce acute episodes.
This study aimed to further test and validate the CTR externally as a modifiable measure of exacerbation risk in a different population, specifically a predominantly Medicare population from the Humana research database. A second objective was to assess the measure’s capability to assess risk using only pharmacy claims, so that exacerbation reduction efforts may be implemented in Medicare Part D programs.
Methods
Objectives
The primary objective of this study was to externally test and validate the ratio of controller (maintenance) medication to total COPD medication as a modifiable measure of COPD exacerbation risk within a commercial and Medicare Advantage (including Medicare Advantage Part D plan) population using a sample of patients from Humana’s claims database with medical and pharmacy coverage that was demographically different from the sample from which the measure was originally derived and validated. As a secondary objective, only the Medicare Part D plan population was used, which excluded risk factors derived from medical claims (pharmacy-only model).
Study Population
Eligible patients were aged ≥ 40 years and had ≥ 1 inpatient hospitalization with diagnosis of COPD or ≥ 2 physician’s office, emergency department (ED), or urgent care visits with a COPD diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 491.xx, 492.xx, or 496.xx) between January 1, 2007, and December 31, 2012, and ≥ 1 dispensing of an inhaled COPD medication (Appendix A, available in online article) or oral theophylline during the baseline period. All patients were required to have at least 2 years of continuous eligibility after the index date. Patients were excluded if they had at least 1 claim during the baseline period with a diagnosis of asthma (ICD-9-CM code 493.xx). Subjects with a diagnosis of cancer (ICD-9-CM codes 140.xx-172.xx, 174.xx-209.3x, or 209.7x) were also excluded to match the original validation study (Figure 1).11
FIGURE 1.
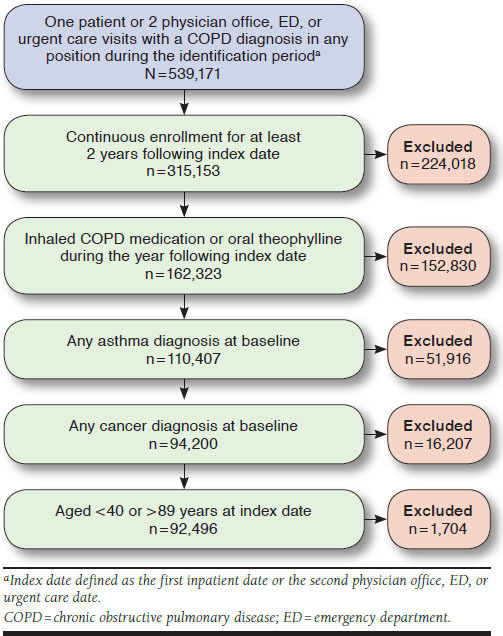
Study Flow Diagram
Study Design
This retrospective study was conducted using the Humana research datasets for the period spanning from January 1, 2007, through December 31, 2014, to test CTR as a measure of COPD exacerbation risk in a group of fully insured commercial and Medicare patients with COPD, based on predictive models previously tested.12 CTR was calculated as the following:
Total units of controller medication (canisters) dispensed ÷ (Total units of controller medication [canisters] dispensed + Total rescue medication [albuterol- and ipratropium-containing medications] dispensed).
The index date was defined as the admitting date of the first hospitalization or the second outpatient, ED, or urgent care visit for which a COPD diagnosis was recorded in the claim. The baseline period was 12 months following the COPD event. The at-risk period consisted of an additional 12-month period following the end of the baseline period (Figure 2).
FIGURE 2.
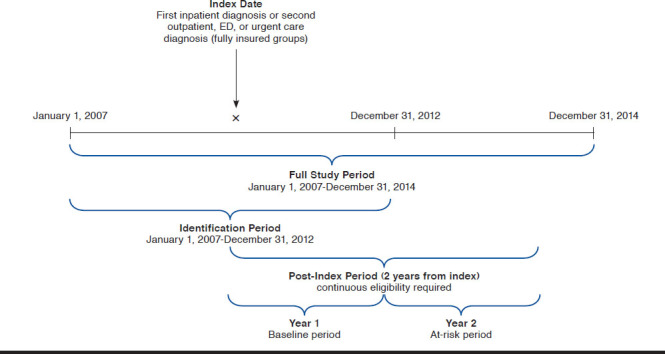
Study Design
Data Sources
The data sources for this study included member enrollment, medical, and pharmacy data entries from Humana’s claims database. Information from these different data entries were linked for each member, using a unique encrypted identifier included in all data sources. This study did not meet the definition of human subjects research and was therefore not subject to the Department of Health and Human Services protection of human subjects regulation. The Humana claims data are fully compliant with the Health Insurance Portability and Accountability Act (HIPAA) of 1996.
Outcomes and Assessments
The primary endpoint was the occurrence of COPD exacerbations during the 1-year at-risk period. Separate models were used for moderate and severe (hospitalized) exacerbations. Moderate exacerbations were defined as outpatient visits or ED visits that did not result in a hospital admission with a diagnosis of COPD (ICD-9-CM codes 491.xx [except 491.20], 492.xx, and 496.xx), either as a primary diagnosis or as a secondary diagnosis, with a primary diagnosis of respiratory failure (ICD-9-CM codes 518.81, 518.82, or 518.84) and a dispensing for an oral corticosteroid ± 7 days from the encounter.13,14 Severe exacerbations were defined as an inpatient hospital stay with a diagnosis of COPD (ICD-9-CM codes 491.xx [except 491.20], 492.xx, or 496.xx), either as a primary diagnosis or as a secondary diagnosis with a primary diagnosis of respiratory failure (ICD-9-CM codes 518.81, 518.82, or 518.84).
Similar models that excluded risk factors derived from medical claims (pharmacy-only model, Medicare Part D) were also assessed.
Statistical Analyses
The association between exacerbations and baseline risk factors, including CTR, was assessed using logistic regression models. Continuous (0.1-0.9) and dichotomized (i.e, ≥ 0.3, ≥ 0.4, ≥ 0.5, ≥ 0.6, ≥ 0.7, ≥ 0.8, or ≥ 0.9) CTR were analyzed. To test the performance of the CTR, this study included all risk factors identified in the final models from a previous study, where applicable.12 Variables in the risk model (age, exacerbation history, COPD and concomitant medication use, and comorbidities) and the CTR were assessed during the baseline period (year 1) and used to predict the exacerbation risk during the follow-up year (year 2). Separate logistic models were estimated for predefined subgroups based on baseline exacerbation history and use of COPD medications: (a) no exacerbation, (b) moderate exacerbation, (c) severe exacerbation, (d) no severe exacerbation, (e) ≥ 1 canister of maintenance or rescue medication dispensed, and (f) ≥ 1 canister of maintenance medication dispensed. The association between each factor and an exacerbation was presented as an odds ratio (OR) with a 95% confidence interval (CI). Statistical significance was assessed at an alpha level of 0.05.
Multicollinearity was assessed by examining correlations between risk factors and by calculating the variance inflation factor from the models. Exacerbation status during the at-risk period was classified as none versus any, in addition to none versus moderate versus severe. Mean, standard deviation (SD), median, minimum, and maximum values of CTR were compared between exacerbation severities. The proportion of patients with any exacerbation was compared between dichotomized CTR groups, initially using a cut-point of ≥ 0.3, based on a previous study.12 Performance of the model was assessed using the c-statistic, a goodness-of-fit measure for dichotomous outcomes in logistic regression models. Values > 0.7 indicate a good model; values > 0.8 indicate a strong model; and a value of 1 represents a model that predicts perfectly the likelihood that a patient will experience an exacerbation. Receiver operating characteristics (ROC) analysis was conducted to determine the optimal dichotomization of CTR, which was included in subsequent analyses. All statistical analyses were conducted using SAS Enterprise Guide version 7.1 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
A total of 92,496 patients were identified. Detailed baseline characteristics, including the presence of risk factors are presented in Table 1. The mean (SD) age of the patients was 69 (9.2) years, and 52.6% were female. The majority (96.2%) were Medicare members who resided in the South (64.9%) or Midwest (23.3%) regions. During the baseline period, 14.0% and 11.2% of patients had ≥ 1 moderate or severe exacerbations, respectively. Chronic airway obstruction was the most prevalent type of COPD diagnosis (67.7%), followed by chronic bronchitis (26.5%) and emphysema (5.8%; Table 1).
Table 1.
Baseline Characteristics and Risk Factors
| Baseline Exacerbation Status | ||||
|---|---|---|---|---|
| Measurea | All (N = 92,496) | None (n = 72,856) | Any Moderate (n = 12,930) | Any Severe (n = 10,378) |
| Demographic | ||||
| Age, mean (± SD) | 69.00 (±9.2) | 68.98 (±9.3) | 68.53 (±8.6) | 69.74 (±8.8) |
| Female | 48,668 (52.6) | 38,403 (52.7) | 6,881 (53.2) | 5,300 (51.1) |
| U.S. region | ||||
| Northeast | 2,046 (2.2) | 1,586 (2.2) | 297 (2.3) | 266 (2.6) |
| Midwest | 21,577 (23.3) | 16,615 (22.8) | 3,210 (24.8) | 2,752 (26.5) |
| South | 60,057 (64.9) | 47,612 (65.4) | 8,270 (64.0) | 6,433 (62.0) |
| West | 8,816 (9.5) | 7,043 (9.7) | 1,153 (8.9) | 927 (8.9) |
| Medicare insurance | 88,956 (96.2) | 69,862 (95.9) | 12,531 (96.9) | 10,179 (98.1) |
| H1N1 flu season overlapped with at-risk period | 19,242 (20.8) | 14,972 (20.6) | 2,560 (19.8) | 2,565 (24.7) |
| Medical | ||||
| Baseline exacerbations | ||||
| 1 moderate | 8,475 (9.2) | 0 | 8,475 (65.5) | 1,889 (18.2) |
| ≥ 2 moderate | 4,455 (4.8) | 0 | 4,455 (34.5) | 1,779 (17.1) |
| 1 severe | 6,172 (6.7) | 0 | 1,953 (15.1) | 6,172 (59.5) |
| ≥ 2 two severe | 4,206 (4.5) | 0 | 1,715 (13.3) | 4,206 (40.5) |
| Type of COPD diagnosis | ||||
| Chronic bronchitis (491.xx) | 24,551 (26.5) | 16,840 (23.1) | 4,958 (38.3) | 4,753 (45.8) |
| Emphysema (492.xx) | 5,344 (5.8) | 4,277 (5.9) | 658 (5.1) | 586 (5.6) |
| Chronic airway obstruction (496.xx) | 62,601 (67.7) | 51,739 (71.0) | 7,314 (56.6) | 5,039 (48.6) |
| Comorbidities | ||||
| Respiratory infection | 47,220 (51.1) | 32,701 (44.9) | 8,923 (69.0) | 8,869 (85.5) |
| Congestive heart failure | 24,316 (26.3) | 16,642 (22.8) | 3,745 (29.0) | 5,753 (55.4) |
| Sleep apnea | 13,848 (15.0) | 10,101 (13.9) | 2,185 (16.9) | 2,378 (22.9) |
| Risk smoker | 38,675 (41.8) | 27,627 (37.9) | 7,195 (55.6) | 6,518 (62.8) |
| Procedures | ||||
| Flu vaccine | 41,469 (44.8) | 32,347 (44.4) | 6,247 (48.3) | 4,587 (44.2) |
| Chronic oxygen therapy | 23,868 (25.8) | 14,651 (20.1) | 5,788 (44.8) | 5,922 (57.1) |
| Nebulizer | 13,448 (14.5) | 8,376 (11.5) | 3,356 (26.0) | 2,992 (28.8) |
| Spirometry | 28,542 (30.9) | 20,517 (28.2) | 5,469 (42.3) | 4,274 (41.2) |
| Medications | ||||
| COPD medications | ||||
| CTR, mean (± SD)b | 0.42 (±0.4) | 0.41 (±0.4) | 0.43 (±0.3) | 0.42 (±0.4) |
| No controller medication | 37,097 (40.1) | 31,319 (43.0) | 3,447 (26.7) | 3,068 (29.6) |
| No controller medication and ≥ 4 canisters of rescue medication | 11,042 (11.9) | 8,879 (12.2) | 1,436 (11.1) | 1,102 (10.6) |
| ≥ 1 dispensing for oral corticosteroids | 39,680 (42.9) | 23,167 (31.8) | 12,917 (99.9) | 7,262 (70.0) |
| ≥ 4 dispensing for oral corticosteroids | 6,847 (7.4) | 2,364 (3.2) | 3,982 (30.8) | 2,184 (21.0) |
| ≥ 1 dispensing for theophylline | 3,013 (3.3) | 1,771 (2.4) | 905 (7.0) | 748 (7.2) |
| Concomitant medications | ||||
| Beta-blockers | 38,284 (41.4) | 29,729 (40.8) | 4,719 (36.5) | 5,450 (52.5) |
| Diuretics | 38,982 (42.1) | 29,246 (40.1) | 5,600 (43.3) | 6,179 (59.5) |
| Statins | 50,871 (55.0) | 40,377 (55.4) | 6,578 (50.9) | 5,835 (56.2) |
| Antidepressants | 33,665 (36.4) | 25,603 (35.1) | 5,111 (39.5) | 4,633 (44.6) |
| Rx-risk score, mean (± SD) | 7.25 (±3.1) | 7.01 (±3.0) | 7.79 (±3.0) | 8.79 (±3.1) |
aAll values given as n (%), unless otherwise stated.
bUnits of controller medication/(units of controller medication + units of rescue medication). One unit is equivalent to 1 canister. For medications not dispensed in canisters,
1 unit is equivalent to a 30-day supply.
COPD = chronic obstructive pulmonary disorder; CTR = COPD treatment ratio; SD = standard deviation.
Performance of the CTR
After initiation of the risk model, every increase of 0.1 (from 0-1) in the CTR was associated with a 1.2% reduction in any exacerbation (adjusted OR = 0.988; 95% CI = 0.978-0.999) and a 2.3% reduction in severe exacerbations (OR = 0.977; 95% CI = 0.963-0.991). Overall, the CTR performed well in predicting future COPD exacerbations, especially severe exacerbations (Table 2), with c-statistics of 0.748 and 0.801 for any (moderate/severe) and severe exacerbations only, respectively. These trends were consistent with those observed in previous validations of the CTR model.11,12
Table 2.
Logistic Models for COPD Exacerbations
| CTR Measure | Model Specifications | OR (95% CI) | |||
|---|---|---|---|---|---|
| Any | Moderate | Severe | |||
| Continuous | Full sample, all predictors | CTR | 0.988 (0.978-0.999) | 0.992 (0.981-1.004) | 0.977 (0.963-0.991) |
| c-statistic | 0.748 | 0.761 | 0.801 | ||
| Continuous | Subsample, no severe exacerbation at baseline, all predictors | CTR | 0.985 (0.974-0.997) | 0.988 (0.975-1.001) | 0.974 (0.958-0.991) |
| c-statistic | 0.717 | 0.736 | 0.754 | ||
| Continuous | Full sample, medical claim predictors removed (pharmacy-only model) | CTR | 0.973 (0.963-0.983) | 0.982 (0.971-0.993) | 0.953 (0.940-0.966) |
| c-statistic | 0.709 | 0.735 | 0.728 | ||
| Dichotomous cut-point 0.7a | Full sample, all predictors | CTR | 0.991 (0.936-1.049) | 1.025 (0.961-1.093) | 0.921 (0.852-0.995) |
| c-statistic | 0.748 | 0.761 | 0.801 | ||
| Dichotomous cut-point 0.7a | Full sample, medical claim predictors removed (pharmacy-only model) | CTR | 0.939 (0.888-0.992) | 0.996 (0.935-1.060) | 0.833 (0.774-0.897) |
| c-statistic | 0.709 | 0.734 | 0.727 | ||
aOR given for CTR ≥ 0.7 vs. < 0.7.
CI = confidence interval; COPD = chronic obstructive pulmonary disorder; CTR = COPD treatment ratio; OR = odds ratio.
Regression results from a full-sample logistic model that excluded medical claim predictors (pharmacy-only model) showed that an increase of 0.1 (from 0-1) in the CTR was associated with a 2.7% (OR = 0.973; 95% CI = 0.963-0.983) reduction in any exacerbation; 1.8% (OR = 0.982; 95% CI = 0.971-0.993) reduction in moderate exacerbation; and 4.7% (OR = 0.953; 95% CI = 0.940-0.966) reduction in severe exacerbation (Table 2). C-statistics for any, moderate, and severe exacerbations were 0.709, 0.735, and 0.728, respectively.
The ROC analysis of dichotomized CTR suggested 0.7 as the optimal cut-point for all 3 models (any, moderate, or severe exacerbation; Table 3), with the closest values for sensitivity and specificity for any of the exacerbations model at 0.6 and 0.55, respectively. When the full model was reestimated with CTR entered as a dichotomous predictor with a cut-point of 0.7, the adjusted ORs (95% CI) for patients with a CTR ≥ 0.7 versus those with a CTR < 0.7 were 0.991 (0.936-1.049), 1.025 (0.961-1.093), and 0.921 (0.852-0.995) for the any, moderate, and severe exacerbation models, respectively (Table 2). In the full model, patients obtaining a CTR ≥ 0.7 had a 7.9% (OR = 0.921; 95% CI = 0.852-0.995) lower risk of a severe exacerbation, compared with those with a CTR < 0.7. A 16.7% (OR = 0.833; 95% CI = 0.774-0.898) reduction was reported in the pharmacy-only model. Similar results were observed in all predefined subgroups (Appendix B, available in online article).
Table 3.
ROC Analysis to Determine Optimal Cut-Point for CTRa
| Total Accuracy | Sensitivity | Specificity | PPV | NPV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTR | Any | Severe | Any | Severe | Any | Severe | Any | Severe | Any | Severe |
| 0.1 | 0.77 | 0.87 | 0.03 | 0.03 | 0.99 | 0.99 | 0.38 | 0.25 | 0.78 | 0.88 |
| 0.2 | 0.76 | 0.85 | 0.09 | 0.09 | 0.95 | 0.95 | 0.34 | 0.21 | 0.79 | 0.88 |
| 0.3 | 0.74 | 0.82 | 0.15 | 0.17 | 0.91 | 0.91 | 0.33 | 0.20 | 0.79 | 0.89 |
| 0.4 | 0.71 | 0.77 | 0.26 | 0.28 | 0.83 | 0.83 | 0.31 | 0.19 | 0.80 | 0.89 |
| 0.5 | 0.63 | 0.66 | 0.41 | 0.44 | 0.69 | 0.69 | 0.27 | 0.16 | 0.80 | 0.90 |
| 0.6 | 0.60 | 0.62 | 0.50 | 0.53 | 0.63 | 0.63 | 0.28 | 0.16 | 0.82 | 0.91 |
| 0.7 | 0.56 | 0.56 | 0.60 | 0.63 | 0.55 | 0.55 | 0.28 | 0.16 | 0.83 | 0.92 |
| 0.8 | 0.52 | 0.50 | 0.70 | 0.73 | 0.47 | 0.47 | 0.27 | 0.16 | 0.85 | 0.93 |
| 0.9 | 0.48 | 0.45 | 0.78 | 0.80 | 0.40 | 0.40 | 0.27 | 0.15 | 0.86 | 0.93 |
aCTR: zero excluded.
CTR = COPD treatment ratio; NPV = negative predictive value; PPV = positive predictive value; ROC = receiver operating characteristics.
Discussion
This report presents the external test and validation of the CTR as a modifiable measure of exacerbation risk in a different population, specifically using data from Humana’s Medicare and commercial database. The predictive properties of the CTR based on claims data have been reported previously.11,12
Overall, although small changes in risk reduction were observed for moderate exacerbation events, the CTR in this study performed well in predicting severe exacerbation risk using claims data, with c-statistics of 0.748 and 0.801 for any (moderate/severe) and severe exacerbations, respectively. This compares favorably with c-statistics reported in other risk models that used clinical information.7,10,15-17 In this study, patients with a CTR ≥ 0.7 were 8% less likely to experience severe exacerbations than those with a CTR < 0.7. Of note, the cut-point of 0.7 identified in this study was much higher than the cut-point reported in the previous studies based on the MarketScan database (0.3),11,12 but it was similar to that observed in other studies.7,10,18-21 Any differences observed in cut-points may be due to differences in patient populations, for example, between those patients who were mostly commercially insured and the patients in this study who were predominantly enrolled in Medicare Advantage plans. This sample was older, reported a higher proportion of males, was very different geographically with a preponderance of cases in the South, and had a different split of COPD etiology and a significantly higher proportion of multiple severe exacerbations in the baseline period. Therefore, while the CTR proves to be a significant predictor, the cut-point may vary depending on the sample used. Further studies are needed to help us better understand the optimal cut-point for clinical applications.
The CTR remained a robust predictor when pharmacy-only claims data were considered. An increase of 0.1 in the CTR was associated with a 3%, 2%, and 5% reduction in any, moderate, and severe exacerbations, respectively. Compared with patients with CTR < 0.7, patients with CTR ≥ 0.7 were 6% less likely to experience any exacerbation and 17% less likely to experience severe exacerbation. The stronger effects in the pharmacy-only models came at the cost of a minimal loss of precision in the overall model performance assessed by c-statistics, indicating that the CTR was capable of capturing much of the underlying risk only observable in medical claims, which may allow the medication ratios based on recent patient histories to be used for better assessment of exacerbation risk.
A patient’s previous history of exacerbation has been demonstrated to strongly predict the occurrence of future exacerbation across all Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages.6,22-24 Numerous additional studies have confirmed that previous COPD exacerbation frequency is a risk factor for future exacerbation independent of COPD severity and other clinical manifestations.23,25,26 A previous study demonstrated that previous moderate exacerbations were a risk factor for a subsequent first severe exacerbation; however, regardless of exacerbation history, the CTR was associated with a reduced risk of first severe exacerbation.12 These results are in line with the recent GOLD report, which places greater emphasis on symptoms and frequency of exacerbations when assessing disease burden and impact.1
Several other exacerbation risk models have been developed based on various risk factors7,10,15-28; however, CTR is the only measure of COPD exacerbation risk that uses readily accessible medical data, specifically health care insurance claims. A number of models based on primary care data have been described and have successfully identified those patients with COPD at highest risk of exacerbation, as well as the risk factors involved.22-24,26 Nevertheless, it is possible that some variables may be underreported, influencing the representativeness of the study sample. Furthermore, these models rely on data from medical records, which are often not easily accessible. CTR has now been validated using several health insurance claims databases among different patient populations and remains as a robust risk model. While many of the other COPD measurement tools for evaluating exacerbation risk have also been validated, they have tended to use smaller validation cohorts and have not necessarily undergone external validation.7,16-18,24,27 Going beyond validation, based on the results of this study, a CTR of ≥ 0.7 may be a useful target to help reduce the risk of severe exacerbations and improve COPD management.
Limitations
Certain limitations should be noted in this study, which are associated with the use of a claims database. First, the identification of exacerbation events was based on definitional algorithms using health insurance claims data that, although widely used in such studies, have not been validated. Presumably, the claim-based definitional algorithms would have < 100% performance. Therefore, there may be misclassification errors for the outcome variables. Second, the risk factors for the multivariable analysis were also limited to the available claims data, which alone may not satisfactorily predict COPD exacerbation. An ICD-9-CM diagnosis code on a medical claim is not an attestation that the patient has the diagnosis, since the code may represent a rule-out diagnosis or may be recorded incorrectly. Finally, since the results of this study were based on members of the Humana database, they may not be generalizable to the U.S. population.
Conclusions
This study has further validated the CTR as a modifiable measure of COPD exacerbation risk in a large Medicare and commercial population. CTR remained a robust predictor of COPD severe exacerbation risk when pharmacy-only claims data were considered, capable of capturing much of the underlying risk for hospitalization events only observable in medical claims, which would allow for more timely assessment of risk. The use of CTR may help predict the risk of future COPD exacerbations, especially severe exacerbations, and since CTR is a modifiable measure, it could be used as a quality of care measure.
ACKNOWLEDGMENTS
Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing, and referencing) was provided by Rachel Edwards, PhD, of Fishawack Indicia, United Kingdom, and was funded by GSK.
APPENDIX A. List of Inhaled COPD Medications
| Drug Group | Drug |
|---|---|
| Short-acting beta2-agonists | Albuterol |
| Levalbuterol | |
| Metaproterenol | |
| Pirbuterol | |
| Terbutaline | |
| Oral corticosteroids | Betamethasone |
| Cortisone acetate | |
| Dexamethasone | |
| Hydrocortisone | |
| Me thylpre dni solone | |
| Paramethasone | |
| Prednisolone acetate | |
| Prednisolone sodium phosphate | |
| Prednisolone | |
| Prednisone | |
| Triamcinolone acetonide | |
| Triamcinolone diacetate | |
| Triamcinolone | |
| Fixed-dose combinations (long acting) | Budesonide-formoterol |
| Fluticasone-salmeterol | |
| Mometasone-formoterol | |
| Long-acting beta-agonist | Arformoterol |
| Formoterol | |
| Indacaterol | |
| Salmeterol | |
| Inhaled corticosteroids | Beclomethasone |
| Budesonide | |
| Ciclesonide | |
| Flunisolide | |
| Fluticasone | |
| Mometasone | |
| Triamcinolone | |
| Phosphodiesterase 4 inhibitors | Roflumilast |
| Short-acting muscarinic agonists | Ipratropium bromide |
| Ipratropium bromide HFA | |
| Long-acting muscarinic agonists | Aclidinium bromide |
| Tiotropium bromide monohydrate | |
| Fixed-dose combination (short acting) | Ipratropium bromide/albuterol |
HFA = HFA-134a (1,1,1,2-tetrafluoroethane).
APPENDIX B. Logistic Models for COPD Exacerbations: Dichotomous Measure of CTR Cut-Point of 0.7 According to Predefined Subgroups During Baseline Period
| Model Specifications | All Predictors | Excluding Medical Claim Predictors (Pharmacy-Only Model) | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||||||
| Any | Moderate | Severe | Any | Moderate | Severe | ||
| No exacerbation | CTR | 0.974 (0.906-1.047) | 0.996 (0.918-1.082) | 0.909 (0.818-1.009) | - | - | - |
| c-statistic | 0.674 | 0.681 | 0.733 | - | - | - | |
| Moderate exacerbation | CTR | 1.086 (0.948-1.244) | 1.094 (0.949-1.260) | 1.048 (0.853-1.288) | - | - | - |
| c-statistic | 0.687 | 0.691 | 0.753 | - | - | - | |
| Severe exacerbation | CTR | 0.996 (0.879-1.129) | 1.076 (0.928-1.247) | 0.920 (0.802-1.057) | - | - | - |
| c-statistic | 0.716 | 0.767 | 0.712 | - | - | - | |
| No severe exacerbation | CTR | 0.996 (0.934-1.062) | 1.019 (0.949-1.094) | 0.936 (0.852-1.028) | 0.960 (0.902-1.022) | 0.997 (0.929-1.069) | 0.874 (0.797-0.959) |
| c-statistic | 0.717 | 0.735 | 0.754 | 0.685 | 0.712 | 0.689 | |
| ≥ 1 controller or rescue medication | CTR | 0.991 (0.936-1.049) | 1.025 (0.961-1.093) | 0.920 (0.852-0.995) | 0.939 (0.888-0.992) | 0.996 (0.935-1.060) | 0.833 (0.774-0.897) |
| c-statistic | 0.748 | 0.761 | 0.801 | 0.709 | 0.734 | 0.727 | |
| ≥ 1 controller medication | CTR | 0.989 (0.935-1.047) | 1.029 (0.965-1.097) | 0.920 (0.852-0.995) | 0.945 (0.894-0.999) | 1.004 (0.943-1.069) | 0.840 (0.780-0.905) |
| c-statistic | 0.749 | 0.758 | 0.806 | 0.712 | 0.73 | 0.738 | |
CI = confidence interval; COPD = chronic obstructive pulmonary disorder; CTR = COPD treatment ratio; OR = odds ratio.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease . 2018 global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Available at: http://goldcopd.org/gold-reports/. Accessed October 11, 2018.
- 2.Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 2007;29(6):1224-38. [DOI] [PubMed] [Google Scholar]
- 3.Andersson F, Borg S, Jansson SA, et al. The costs of exacerbations in chronic obstructive pulmonary disease (COPD). Respir Med. 2002;96(9):700-08. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31-45. [DOI] [PubMed] [Google Scholar]
- 5.Pasquale MK, Sun SX, Song F, Hartnett HJ, Stemkowski SA. Impact of exacerbations on health care cost and resource utilization in chronic obstructive pulmonary disease patients with chronic bronchitis from a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis. 2012;7:757-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128-38. [DOI] [PubMed] [Google Scholar]
- 7.Marin JM, Carrizo SJ, Casanova C, et al. Prediction of risk of COPD exacerbations by the BODE index. Respir Med. 2009;103(3):373-78. [DOI] [PubMed] [Google Scholar]
- 8.Mullerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999-1007. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Aymerich J, Farrero E, Felez MA, et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58(2):100-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SD, Huang MS, Kang J, et al. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Respir Med. 2014;108(4):600-08. [DOI] [PubMed] [Google Scholar]
- 11.Stanford RH, Nag A, Mapel DW, et al. Validation of a new risk measure for chronic obstructive pulmonary disease exacerbation using health insurance claims data. Ann Am Thorac Soc. 2016;13(7):1067-75. [DOI] [PubMed] [Google Scholar]
- 12.Stanford R, Nag A, Mapel D, et al. Claims-based risk model for first severe COPD exacerbation. Am J Manag Care. 2018;24(2):e45-e53. [PubMed] [Google Scholar]
- 13.Mapel DW, Schum M, Lydick E, Marton JP.. A new method for examining the cost savings of reducing COPD exacerbations. Pharmacoeconomics. 2010; 28:733-49. [DOI] [PubMed] [Google Scholar]
- 14.Abudagga A, Sun SX, Tan H, Solem CT. Exacerbations among chronic bronchitis patients treated with maintenance medications from a US managed care population: an administrative claims data analysis. Int J Chron Obstruct Pulmon Dis. 2013;8:175-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertens LC, Reitsma JB, Moons KG, et al. Development and validation of a model to predict the risk of exacerbations in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2013;8:493-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerkhof M, Freeman D, Jones R, Chisholm A, Price DB. Predicting frequent COPD exacerbations using primary care data. Int J Chron Obstruct Pulmon Dis. 2015;10:2439-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Make BJ, Eriksson G, Calverley PM, et al. A score to predict short-term risk of COPD exacerbations (SCOPEX). Int J Chron Obstruct Pulmon Dis. 2015;10:201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brusse-Keizer M, van der Palen J, van der Valk P, Hendrix R, Kerstjens H. Clinical predictors of exacerbation frequency in chronic obstructive pulmonary disease. Clin Respir J. 2011;5(4):227-34. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Sidro P, Naval E, Martinez Rivera C, et al. The CAT (COPD Assessment Test) questionnaire as a predictor of the evolution of severe COPD exacerbations. Respir Med. 2015;109(12):1546-52. [DOI] [PubMed] [Google Scholar]
- 20.Miravitlles M, Garcia-Sidro P, Fernandez-Nistal A, et al. The chronic obstructive pulmonary disease assessment test improves the predictive value of previous exacerbations for poor outcomes in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:2571-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motegi T, Jones RC, Ishii T, et al. A comparison of three multidimensional indices of COPD severity as predictors of future exacerbations. Int J Chron Obstruct Pulmon Dis. 2013;8:259-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-ani S, Spigt M, Hofset P, Melbye H. Predictors of exacerbations of asthma and COPD during one year in primary care. Fam Pract. 2013;30(6):621-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santibanez M, Garrastazu R, Ruiz-Nunez M, et al. Predictors of hospitalized exacerbations and mortality in chronic obstructive pulmonary disease. PloS One. 2016;11(6):e0158727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montserrat-Capdevila J, Godoy P, Marsal JR, Barbe F. Predictive model of hospital admission for COPD exacerbation. Respir Care. 2015;60(9):1288-94. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Xiang P, Zhang E, et al. Predictors of exacerbation frequency in chronic obstructive pulmonary disease. Eur J Med Res. 2014;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullerova H, Shukla A, Hawkins A, Quint J. Risk factors for acute exacerbations of COPD in a primary care population: a retrospective observational cohort study. BMJ Open. 2014;4(12):e006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briggs AH, Baker T, Risebrough NA, et al. Development of the Galaxy Chronic Obstructive Pulmonary Disease (COPD) Model using data from ECLIPSE: internal validation of a linked-equations cohort model. Med Decis Making. 2017;37(4):469-80. [DOI] [PubMed] [Google Scholar]
- 28.Sadatsafavi M, Sin DD, Zafari Z, et al. The association between rate and severity of exacerbations in chronic obstructive pulmonary disease: an application of a joint frailty-logistic model. Am J Epidemiol. 2016;184(9):681-89. [DOI] [PMC free article] [PubMed] [Google Scholar]


