Abstract
Background
Atherogenic Index of Plasma (AIP) is a reliable predictor of coronary artery disease. There is paucity of data on AIP and its correlates among patients with type 2 diabetes (T2D) in Uganda.
Objective
To assess the profile of AIP and its cardiovascular risk factor correlates among patients with T2D in Uganda.
Methods
This was a cross-sectional study conducted in 8 health facilities with established T2D clinics in Central-Uganda. The study enrolled 500 patients aged 40 to 79 years. Data was collected on socio-demographic characteristics, lipid profile and glycated haemoglobin (HbA1c). The AIP was derived using log (triglycerides/high-density cholesterol) and further categorised as low cardiovascular disease (CVD) risk if the AIP was <0.1, intermediate risk (0.1-0.24) and high risk (≥0.24). Cardiovascular risk factors were defined according to international guidelines.
Stata version 14 was used to analyse data, Pearson correlation analyses were conducted. Statistical significance was set at p<0.05.
Results
There were 389(77.4%) females with a mean age of 55.07±8. 979 years. Low-risk was found in 43.6%, intermediate risk in 20.2% and high risk in 36.2% of the participants. AIP significantly correlated with waist circumference (r=0.1095, p<0.0147), waist-hip ratio (r=0.1926, p<0.001), Casteri Risk Index I (r=0.506, r=<0.001), Casteri Risk Index II (r=0.246, p<0.001) and atherogenic coefficient (r=0.186, p<0.001). Insignificant correlation was observed between AIP and fasting blood sugar (r=0.017, p=0.7042), HBA1C (r=0.0108, p=0.8099) and diabetes duration (r=0.0445, p=0.32)
Conclusions
AIP is significantly elevated and correlated with cardiovascular risk factors in patients with T2D. In clinical management, this may be a useful tool in risk stratifying patients with T2D.
Keywords: Atherogenic index of plasma, Type 2 diabetes, cardiovascular risk factors, Uganda
Introduction
Diabetes mellitus (DM) is an established risk factor for cardiovascular disease (CVD)1, and patients with DM are at a two to four-fold higher cardiovascular risk, including myocardial infarction, unstable angina, stroke, and heart failure2. All of the above have arisen interest in CVD preventive strategies by the use of non-invasive methods, such as risk scores.3 The most common approach is to consider DM as a CVD equivalent and, therefore, to treat patients with DM in a similar way to those who required secondary CVD prevention4. However, this approach has been disputed as all patients with T2D do not have the same CVD risk5,6 and since other potentially important factors within the context of DM, such as DM duration, presence of albuminuria, and comorbidities, should be taken into consideration. Moreover, diabetic dyslipidaemia which is major orchestrator of CVD is characterised by elevated triglyceride(TGs), reduced high density cholesterol(HDL) and small LDL particles 2 Diabetic dyslipidaemia in T2D is comprised of quantitative, qualitative and kinetic abnormalities of the lipid parameters 7 In clinical practice, it is the quantitative abnormality that is routinely assessed through estimation of lipid parameters which may be normal or subnormal missing the opportunity to tackle qualitative and kinetic abnormalities 8 hence predisposing patients to CVD. The early detection of lipid abnormalities with their management can mitigate the risk of atherogenic cardiovascular and cerebral vascular disorders in T2D patients9. Timely detection of lipid abnormalities can be achieved through quantitative determination of lipid variables and atherogenic cardiovascular indices8. Qualitative akinetic abnormalities characterised by small LDL particles would be indirectly measured by Lipoprotein(a) assays10, but these are not routinely done due to cost and unavailability of test. LDL particle size can be assessed with Atherogenic Index of Plasma. Atherogenic Index of Plasma (AIP)defined as the base 10 logarithm of the ratio of plasma triglyceride (TG) to high density lipoprotein (HDL)11, has been used to assess for small LDL particles and plasma atherogenicity11 .AIP is a major predictor of atherosclerosis and coronary heart disease and reflects the relationship between protective and atherogenic lipoproteins12, AIP is thus an indicator of LDL particle size that is not determined by the usual lipid profile.
It can be easily derived from the usual lipid profile which is done widely among T2D patients in Uganda at no added cost. AIP can be used to stratify T2D patients so that those at high CVD risk can be started on drugs with proven cardiovascular benefit13 such as Sodium Glucose Co Transporter 2 inhibitors (SGLT2) or Glucagon like peptide 1 agonists (GLP-IA) as stipulated by several International guidelines such as the American Diabetes Association Standards of Care on cardiovascular disease and risk management 202213. Currently, the majority of medicine and diagnostic tests essential in the management of type 2 diabetes and CVD are unavailable and unaffordable in Uganda14. So, prevention is key though screening with cheap and available tests such as the lipid profile and its derivative, the Atherogenic Index of Plasma.
Whereas the statin dose intensity and use of ant platelet agents is clear for those who have had a CVD event13 it is not clear for those who are asymptomatic, hence there is need for guidance in this regard so that meagre resources can be used sparingly in a poor resource setting. This study looked at AIP and its cardiovascular risk factor correlates in a Ugandan T2D population.
Knowledge of the profile of AIP and its cardiovascular risk factor correlates is key for CVD risk stratification11,15, so that T2D patients can be started appropriately on drugs with proven cardiovascular benefit to prevent or manage CVD 13 at primary level in our poor resource setting.
Additionally, markers of obesity such as body mass index, waist circumference, waist hip ratio and atherogenic indices have been shown to correlate with a high CVD risk9. Therefore, proof of this correlation with AIP among T2D patients in Uganda will be useful in predicting CAD risk in centres where a lipid profile can't be performed. Therefore, the current study was conducted to assess the profile of Atherogenic Index of Plasma and its cardiovascular risk factor correlates among patients with T2D in Central Uganda.
Materials and Methods
Study Design and Population
This was a cross-sectional study conducted in eight (8) diabetes clinics in Central-Uganda namely; Entebbe grade B hospital, Mengo Hospital, Naguru Hospital, Kasangati Health Center IV, Wakiso Health Center IV, Mpigi Health Center IV, Mityana Hospital and Kawolo Hospital. Detailed description of the methodology has been published elsewhere16. The study was approved by Mengo Hospital Research and Ethics Committee (Approval number MH/REC/100/9-2019) and was registered by Uganda National Council of Science and Technology (Registration number HS2738). We obtained administrative clearance letters from heads of participating hospitals. Written informed consent was obtained from each study participant. We followed the Declaration of Helsinki during the execution of this study.
Data was collected from 500 patients with T2D aged between 40 and 79 years who were consecutively selected from the diabetes registers. Eligible patients were given unique identification numbers derived from the health facility code and registration number.
Socio-demographic data on age, sex, level of education, employment status and smoking were collected by trained study nurses through face-to-face interviews.
Anthropometric, clinical and laboratory measurements were conducted. Details of these measurements have been published elsewhere.16
Atherogenic Index of Plasma
Atherogenic Index of Plasma (AIP) was calculated from Log10(TG/HDLc) ratio. Low CAD risk was when AIP was -0.3-<0.1, medium risk 0.1 -<0.24 and high CAD risk ≥0.24.17
Cardiovascular Risk factors
Cardiovascular risk factors in T2D were defined according to W.H.O 18 and American Diabetes Association Standard of Care Guidelines13. American Diabetes Association Standard of Care 2021 targets for cardiovascular risk factors among T2D were used to define CVD risk factors among our study population13. Elevated glycated haemoglobin was defined ≥ 7%, systolic blood pressure≥ 140mmHg, diastolic blood pressure≥90mmHg, low-density lipoprotein cholesterol ≥ 2.56mmol/l(100mg/dl)13. Additionally, elevated fasting blood sugar was ≥7mmol/, total cholesterol≥5.12mmol/l and triglyerides≥ 1.7mmol/L13. A high waist circumference was defined as ≥88cm for women and ≥102cm for men. We took a waist-hip ratio ≥0.88(women) and ≥0.95(men)and a body mass index >25Kgm2 to be elevated 18. A duration of ≥ 10years, gender was taken as a CVD risk factors3.
Smoking was defined as daily cigarette smoking, family history of premature coronary heart disease was defined as history of CHD in first degree relatives aged < 55 years for males and <65 years for females3.
Castelli Risk Index 1(CRI-1) was estimated as: TC/HDLc ratio, A low risk was defined as <3.515. Castelli Risk Index II(CRI-II) was determined as LDL-c/HDLc ratio. A low-risk was defined <3.015. Atherogenic Coefficient (A.C) was calculated as (TC-HDLc)/HDLc or (Non-HDLc)/HDLc ratio A low risk was defined as < 3.015.
Statistical analysis
The data collected was checked for completeness, coded and entered into EpiData manager version 4.6 and exported to STATA version 14. Continuous variables were described using the mean and standard deviation. Categorical variables were expressed as frequencies and percentages and were compared using Chi-square tests. The correlation between AIP and cardiovascular risk factors was determined by Pearson chi-square correlation analyses. A p-value of less than 0.05 was considered statistically significant.
Results
Socio demographic characteristics
Out of the 500 study participants, 389 (77.4%) were females and the mean age of the participants was 55.07years±8.979. One hundred eight seven (37.4%) were aged between 50 and 59 years. Three hundred twenty-three (64.6%) of the study participants were urban residents. About 442(88.4%) did not have history of premature coronary heart disease death in the family. The majority 448(89.6%) did not take alcohol and 491(98.2%) did not smoke. Thirty-five (7%) of the participants were on lipid lowering medications by the time of our study. About three hundred and sixty-seven (73.4%) of the participants had had DM for less than 10 years while 364(72.8%) were taking ant hypertensive medications. Details are shown in Table 1.
Table 1.
Socio-demographic and clinical characteristics of participants
| Variable | Female (n=389) |
Male(n=111) | All(N=500) | Mean±SD |
| n (%) | n (%) | n (%) | ||
| Age Group | ||||
| 40–49 | 114(29.31) | 30(27.03) | 144(28.8) | |
| 50–59 | 141(36.25) | 46(41.44) | 187(37.4) | |
| 60–69 | 105(26.99) | 28(25.23) | 133(26.6) | |
| >70 | 29(7.46) | 7(6.31) | 36(7.2) | |
| Mean age | 55.076±8.978 | |||
| Residence | ||||
| Urban | 252(64.78) | 71(63.96) | 323(64.6) | |
| Rural | 137(35.22) | 40(36.04) | 177(35.4) | |
|
History of Premature CHD death |
||||
| Yes | 31(7.97) | 5(4.50) | 36(7.20) | |
| No | 337(86.63) | 105(94.59) | 442(88.40) | |
| Don't know | 21(5.40) | 1(0.90) | 22(4.40) | |
| Alcohol intake | ||||
| Yes | 21(5.4) | 13(11.11) | 34(6.8) | |
| No | 358(92.03 | 90(81.08) | 448(89.6) | |
| Quit | 10(2.54) | 8(7.2) | 18(3.6) | |
| Smoking | ||||
| Yes | 1(0.26) | 0.00 | 1(0.2) | |
| No | 386(99.23) | 105(94.59) | 491(98.2) | |
| Quit | 2(0.51) | 6(5.41) | 8(1.6) | |
|
Cholesterol Lowering Medicine use |
||||
| Yes | 24(6.17) | 11(9.91) | 35(7) | |
| No | 365(93.83) | 100(90.09) | 465(93) | |
| Duration of DM | ||||
| <10years | 288(78.47) | 79(21.53) | 367(73.4) | |
| >10years | 101(75.94) | 32(24.06) | 133(26.4) | |
| Use of Hypertensives | ||||
| Yes | 291(74.81) | 73(65.771) | 364(72.8) | |
| No | 98(25.19) | 38(34.23) | 136(27.2) |
Profile of the Atherogenic Index of Plasma
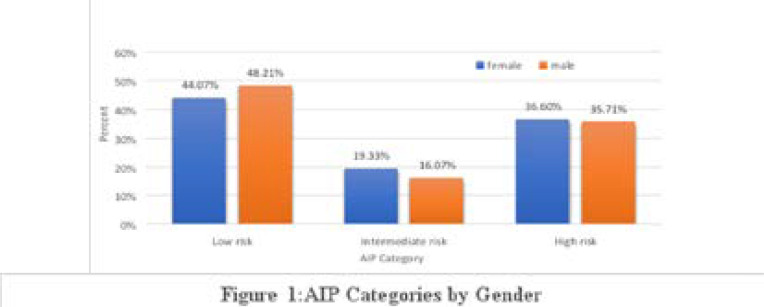
Out of 500 participants,218(43.6%) had low, 101(20.2%) moderate and 181(36.2%) high AIP. The mean AIP was 0.177±0.413. There was no significant gender difference across all categories of AIP as shown in figure 1.
Figure 1.
AIP Categories by Gender
Cardiovascular risk factors among study participants
Three hundred and seventy-nine (75.8%) of the study participants had a BMI ≥25Kg/m2 with a mean of 29.78 Kg/m2 ±5. 778. Two hundred and eighty-four (56.8%) had elevated waist-hip ratio though there was no significant difference in mean waist circumference between females and males though men had a higher mean waist-hip ratio 0.942±0. 106. Elevated systolic blood pressure and diastolic blood pressure was found among 211(42.2%) and 158(31.6%) of participants respectively. However, the mean combined blood pressure across all the study participants, was 137.53/85.62mmhg.
Elevated total cholesterol was found among 290(58%) of the study participants with a mean value of 5.405mmol±1.319 while 393 (78.6%) had elevated LDL with a mean of 3.502mmol/l±1. 181.Seventy-two percent (72%) of the participants had elevated glycated Hemoglobin (HbA1C≥7%). About two thirds had fasting hyperglycemia (fasting blood sugar ≥ 7mmol/l). The mean DM duration was 6.952 ±6.383 years.
Regarding cardiovascular indices,357(71.4%) of the participants had an elevated CRI I and there was no significant gender difference(p=0.818). Two hundred eighty-one (56.2%) of the participants had their A.C raised. Similarly, there was no gender difference noted in this parameter(p=0.524). More details about the CVD risk factors are shown in tables 2 and 3.
Table 2.
Anthropometric and laboratory measurements of Participants (N=500)
| Variable | Female (n=389) |
Male(n=111) | All(N=500) | p-value |
| n (%) | n (%) | n (%) | ||
| Body mass Index | ||||
| <18Kg/m2 | 1(0.26) | 0.00(0) | 1(0.2) | 0.56 |
| 18–24.99Kg/m2 | 70(17.99) | 34(30.63) | 104(20.8) | |
| 25–29.99Kg/m2 | 135(34.7) | 44(39.64 | 179(35.8) | |
| ≥30Kg/m2 | 183(48.59) | 11(9.91) | 200(40) | |
| Waist Circumference | ||||
| <88.8cm | 200(51.47) | 100(90.09) | 300(60) | |
| ≥88.8cm | 189(48.59) | 11(9.91) | 200(40) | |
| Waist Hip Ratio | ||||
| <0.88 | 156(40.17) | 60(54.05) | 216(43.2) | |
| ≥0.88 | 233(59.90) | 51(45.95) | 284(56.8) | |
| SBP | ||||
| <140mmHg | 237(60.93) | 52(46.85) | 289(57.8) | 0.008 |
| ≥140mmHg | 152(39.07) | 59(53.15) | 211(42.2) | |
| DBP | ||||
| <90mmhg | 273(70.18) | 69(62.16) | 342(68.4) | 0.109 |
| ≥90mmhg | 116(29.82) | 42(37.84) | 158(31.6) | |
| T.C | ||||
| <5.13mmol/l | 147(37.79) | 63(56.76) | 210(42) | <0.001 |
| ≥5.13mmol/l | 242(62.21) | 48(43.24) | 290(58) | |
| HDL-c | ||||
| ≥1.3 mmol/l | 342(87.92) | 87(78.38) | 233(46.6) | <0.001 |
| <1.3mmo/l | 47(12.08) | 24(21.62) | 267(53.7) | |
| TG | ||||
| <1.685mmol/l | 159(40.87) | 62(55.86) | 221(44.2) | 0.005 |
| ≥1.685mmo/l | 230(59.13) | 49(44.14) | 279(55.8%) | |
| LDL-c | ||||
| <2.56mmol/l | 66(16.97) | 41(36.94) | 107(21.4) | <0.001 |
| ≥2.56mmol/l | 323(83.03) | 70(63.06) | 393(78.6) | |
| CRI I | ||||
| <3.5 | 110(28.35) | 32(29.46) | 143(28.6) | |
| ≥3.5 | 278(71.65) | 79(70.54) | 357(71.4) | 0.818 |
| CRI II | ||||
| <3.3 | 282(72.94) | 86(76.79) | 369(73.8) | |
| ≥3.3 | 105(27.06) | 26(23.21) | 131(26.2) | 0.415 |
| AC | ||||
| <3.0 | 167(43.04) | 52(46.43) | 219(43.8) | |
| ≥3.0 | 221(56.96) | 60(53.57) | 281(56.2) | 0.524 |
| FBS | ||||
| <7mmol/l | 125(32.13) | 37(33.33) | 162(32.4) | 0.812 |
| ≥7mmo | 264(67.87) | 74(66.67) | 338(67.6) | |
| HbA1C | ||||
| <7% | 112(28.79) | 28(25.23) | 140(28) | |
| ≥7% | 277(71.21) | 83(74.77) | 360(72) | 0.46 |
| AIP | ||||
| Low risk (<0.11) | 171(44.07) | 54(48.21) | 225(45) | |
| Intermediate risk (0.11-<0.24 | 75(19.33) | 18(16.07) | 93(18.6) | |
| High risk≥0.24 | 142(36.6) | 40(35.71) | 182(36.4) | |
n frequency, SBP: Syst olic blood pressure, DBP: Diastolic blood pressure, HbA1c: Glycated haemoglobin, T.C: Total cholesterol, HDL-C: high density lipoprotein cholesterol, TG: Triglycerides, LDL-C: Low density lipoprotein cholesterol, CRI I: Casteri Risk Index I, CRI II: Casteri Risk Index II, AC: Atherogenic Coefficient: FBS: Fasting Blood Sugar: HBA1C: Glycated Hemoglobin, AIP: Atherogenic Index of Plasma
Table 3.
Mean anthropometric, clinical and laboratory measurements of study participants
| Variable | Mean (± SD) | ||
| Female | Male | All | |
| BMI (kg/m2) | 30.25 (5.76) | 28.15 (5.58) | 29.78 (5.78) |
| W.C (cm) | 88.40 (12.66) | 88.88 (11.83) | 88.50 (12.44) |
| W. H. R | 0.89 (0.09) | 0.94 (0.11) | 0.90 (0.09) |
| SBP (mmHg) | 136.19 (19.51) | 141.65 (19.71) | 137.54 (19.66) |
| DBP (mmHg) | 85.19 (11.42) | 87.12 (10.72) | 85.62 (11.29) |
| HBA1C (%) | 8.9 (2.85) | 8.97 (2.73) | 8.92 (2.82) |
| FBS (mmol/l) | 9.59 (5.14) | 9.26 (3.81) | 9.51 (4.88) |
| T.C (mmol/l) | 5.54 (1.29) | 4.92 (1.32) | 5.41 (1.32) |
| HDL-C (mmol/l) | 1.36 (0.37) | 1.23 (0.35) | 1.33 (0.37) |
| LDL-C (mmol/l) | 3.63 (1.16) | 3.07 (1.17) | 3.50 (1.18) |
| TGs (mmol/l) | 2.06 (1.06) | 2.14 (1.76) | 2.08 (1.25) |
| CRI-1 | 4.29 (1.38) | 4.25 (1.54) | 4.28 (1.42) |
| CRI-11 | 2.79 (1.03) | 2.57 (1.02) | 2.74 (1.03) |
| AIP | 0.18 (0.44) | 0.18 (0.321) | 0.18 (0.413) |
| A.C | 3.18 (1.28) | 3.25 (1.16) | 3.28 (1.43) |
| Diabetes Duration (years) | 6.92 (6.21) | 7.07(6.99) | 6.95 (6.38) |
BMI: Body Mass Index: W.C: Waist Circumference: Waist Hip Ratio, SBP: Systolic Blood Pressure, DBP: Diastolic Blood Pressure, HBA-IC: Glycated Hemoglobin, FBS: Fasting Blood Sugar, TC:Total Cholesterol, HDL: High Density Lipoprotein Cholesterol, LDL-C:Low Density Lipoprotein Cholesterol, TGs: Triglycerides, CRI 1: Casteri Risk Index I, CRI II: Casteri Risk Index II, AIP: Atherogenic Index of Plasma: AC: Atherogenic Coefficient:
Correlation between Atherogenic Index of Plasma and cardiovascular risk factors
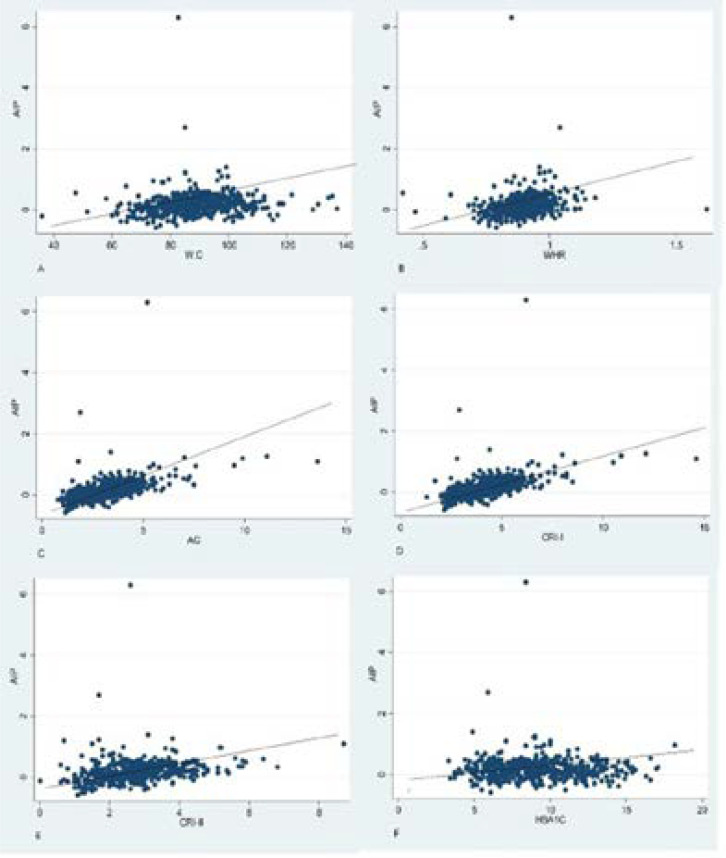
We performed Pearson's correlation analysis to investigate the correlation between AIP and cardiovascular risk factors. AIP positively and significantly correlated with waist circumference (r=0.1095, p<0.0147), waist-hip ratio (r=0.1926, p=0.0000), CRI I (r=0.506, r=<0.001), CRI II (r=0.246, p<0.001) and A.C(r=0.186, p<0.001). Furthermore, weak and insignificant correlation was noted between AIP and sex (r=0.003, p=0.995), BMI (r=0.0349, p=0.436), total cholesterol (r=0.0691, p=0.012), fasting blood sugar (r=0.017, p=0.7042), glycated haemoglobin (r=0.0108, p=0.8099), LDL (r=0.0810, p=0.0705, DM duration (r=0.0445, p=0.32). Details are depicted in table 4 and figure 2.
Table 4.
Correlation between AIP and Cardiovascular Risk Factors
| Parameter | r | P -value |
| Age | 0.0746 | 0.0957 |
| Male Sex | 0.0003 | 0.9950 |
| BMI | 0.0349 | 0.436 |
| Waist circumference | 0.1095 | 0.0147 |
| Waist Hip ratio | 0.1976 | <0.001 |
| Fasting blood sugar | 0.017 | 0.7042 |
| Glycated Hemoglobin | 0.018 | 0.8095 |
| Systolic blood pressure | 0.0457 | 0.3055 |
| Diastolic blood pressure | 0.0093 | 0.8361 |
| Duration of diabetes | 0.0445 | 0.3209 |
| Total cholesterol | 0.0691 | 0.123 |
| LDLc | 0.0810 | 0.07055 |
| Casteri Risk Index I | 0.185 | <0.001 |
| Casteri Risk Index I | 0.127 | <0.004 |
| Atherogenic Coefficient | 0.186 | <0.001 |
BMI=Body Mass Index, LDLc=Low Density Lipoprotein cholesterol, r= Pearson's correlation coefficient
Figure 2.
Correlation of AIP with A) Waist Circumference, B) Waist Hip Ratio, C) Atherogenic Coefficient D) Casteri Risk Index I E) Casteri Risk Index II F) Glycated Hemoglobin
WC=Waist Circumference, WHR=Waist-Hip Ratio, CRI I=Casteri Risk Index I, CRI II=Casteri Risk Index II, HBA1C=Glycated Hemoglobin
Discussion
In this cross-sectional study, we determined the profile of atherogenic index of plasma and its cardiovascular risk factor correlates among patients with T2D in 8 rural and urban clinics in Central Uganda. Our study showed 56.4% of the participants had intermediate and high AIP (atherogenic risk) with no significant difference in distribution across gender. The current study showed that a significant proportion of our patients with T2D had poorly controlled cardiovascular disease risk factors.
Our study showed that AIP was positively and significantly correlated with waist circumference, waist- hip ratio, CRI-I, CRI-II and A.C. Weak and insignificant correlation was noted between AIP and sex, BMI, total cholesterol, fasting blood sugar, glycated haemoglobin and duration of diabetes.
Similar results were shown in one study among adult Africans living with HIV infection by Steve Raul Noumegni et al where 52.4% had intermediate and high atherogenic risk 19.
In a large population-based survey conducted over 7000 subjects in rural Uganda,42% of the individuals had high and intermediate atherogenic risk (20). The differences in study design, population sample, nature of patients studied and specimen collected could explain the differences in the findings between the two studies.
In a study conducted by S. Bhardwaj et al, AIP, CRI 1, CRI II and AC were significantly elevated in angiographically confirmed cases of CAD and contributed significantly to the CAD risk with AIP contributing 31% followed by CRI 1 with 20%, AC 17% and CRI II11. It is not surprising that these cardiovascular indices are elevated in our T2D patients since diabetes portends a very high coronary heart disease risk. Our study has shown a significant correlation between AIP and CRI I, CRI II and AC. These indices signify a high plaque density in the coronary arteries11. Therefore, their elevation among our study participants indicates a high CAD risk that needs urgent tackling in our setting.
Our findings are consistent with a study done in Nigeria by Bolarinwa et al that found a high AIP in 31.4% of diabetic and hypertensive patients attending a tertiary hospital 17. This study is similar to ours in that they were both cross-sectional hospital-based studies with similar patients studied. However, there was a gender difference in terms of the atherogenic risk with females more at risk than men that was attributed to the higher number of women recruited in the study. Our study had a higher number of women but we didn't show any gender difference in the risk probably due to the fact that with a mean age of 55.07±8.979years, women in our study were post-menopausal. It has been shown that before menopause, estrogen is cardiovascular protective and hence in the premenopausal period men tend to have a higher CVD risk than women but this advantage cancels out after menopause 3. In a similar study by Olamoyegun et al, AIP showed no significant difference between sexes in predicting cardiovascular disease risk 15..
Consistent with earlier studies 12,17,21–23, AIP positively and significantly correlated with waist circumference and waist-hip ratio. We didn't not show significant relationship between AIP and BMI similar to a study by Steve Raoul Noumegni et al19. Waist-hip ratio is a better marker of visceral adiposity than BMI which does not differentiate between body fat and lean mass24. Visceral adiposity is associated with increase in free fatty acid levels that cause insulin resistance by reducing insulin signalling at the target tissues. Insulin resistance is a driver of cardiovascular disease 23.
In our study population, more than 50% had diabetic dyslipidaemia characterised by a high LDL (mean 3.502mmol/l±1.181), high triglyceride (mean 2.078mmol/l±1.24) and low HDL (mean 1.33mmol/l±0.372), this type of dyslipidaemia typical of diabetes is highly atherogenic and is not surprising that 56.4% of our study participants had a high plasma atherogenicity as determined by the AIP. This is in tandem with several other studies 12,17,23,25.
Contrary to other studies12,17,23, we didn't show a significant relationship between AIP and LDL despite 78.4% of the study participants having elevated LDL probably the two measures assess different abnormalities in an individual. Whereas the importance of LDL cholesterol in the development of atherosclerosis is well documented, and it being the principal target for prevention of CHD, the heterogeneity of the LDL particles has been recently elucidated 24 Different forms of LDL that include small dense LDL particles and oxidized LDL have been described and these constitute the kinetic abnormalities of LDL other than the quantitative abnormality that is routinely assessed by the usual lipid profile. In one study in Canada, small dense LDL particles predicted the rate of ischaemic heart disease independent of LDL cholesterol, TGs, HDL cholesterol, apo B and the TC/HDL ratio. The same study showed that LDL diameter predicts ischaemic heart disease risk more accurately than traditional lipid variables. Therefore, AIP assesses the small dense LDL particles while the absolute LDL measurement determines the quantitative abnormality. Our finding underpins the utility of assessing for both among our patients with the AIP very crucial in those patients who seem to have normal or low LDL values24.
We have earlier shown that 65.8% of patients with T2D have a high 10 year predicted ASCVD risk16 as determined with the Pooled Cohorts Risks Equation and the current study has shown that 56.4% of our patients have elevated AIP. The 10 -year predicted ASCVD risk assesses the global ASCVD risk i.e., coronary heart disease, ischaemic stroke and peripheral arterial disease risk while AIP is mainly a predictor of coronary artery disease26, which is the main cause of morbidity and mortality among the CVD spectrum in patients with T2D. These findings can be explained by high LDL levels among our patients (mean LDL was 3.5±1.181) and only 7 % of the patients were taking lipid lowering drugs by the time of our study. Therefore, the significant elevation of AIP in more than half of the patients shown in our study means that there is an urgent need for use of high dose statin, SGLT2 inhibitors, GLPA1 agonists, ant platelet agents and other life style changes for primary prevention of CVD13
In our study, AIP was not significantly correlated with systolic and diastolic blood pressure which was in tandem with a study done by Manoj Kumar et al assessing the association of AIP with haemodynamic variables in normotensive and never treated hypertensive subjects. In that study, AIP didn't correlate with arterial Blood pressure but correlated directly and indirectly with arterial stiffness27. In one study, AIP correlated with systolic blood pressure and diastolic blood but there was no significant difference(p=0.06) between hypertensive and non-hypertensive groups which was due to a higher number of normotensive participants22. It should be noted that the majority of our patients had well controlled hypertension which could have explained the insignificant correlation. The current study did not show significant correlation between AIP and measures of glycaemia namely fasting blood sugar and glycated haemoglobin. The reasons for this remain unclear to us. However, our findings are similar to those found by James B Meiggs et al in a study in which there was no association between glycemic control and prevalent cardiovascular disease among 1,539 patients with Non-Insulin Dependent Diabetes Mellitus28
Contrary to our study, Zhang H et al in a large prospective population-based cohort study among 10060 Chinese, showed that an HbA1C of 7-8% was significantly associated with a high CVD risk in patients with baseline ASCVD risk score≥10%29. The difference in outcomes could be due to a larger sample size, follow up period and difference in CVD risk assessment method.
While hyperglycemia is evidently associated with microvascular complications, its role in the causation of large vessel atherosclerosis is controversial28.
Despite the fact that our patients' mean glycated hemoglobin was 8.919±2.818% which is strongly related to CVD30, we didn't show this relationship as causation of ASCVD by hyperglycemia is time dependent31, i.e., more evident when duration of diabetes is ≥10years. In the current study, 73.4% of our patients had had diabetes for less than 10 years with a mean duration of 6.952 ±6.38 years. Additionally, this being a cross sectional study, causal relationship could not be established.
The major strength of this study is that it assessed atherogenic AIP and its cardiovascular risk factor correlates among patients with T2D for the first time in Uganda. Secondly, the study was conducted in one region of the country with limited ethnic variation. However, there are some limitations. The cross-sectional design does not allow to infer a causal-effect relationship between AIP and other cardiovascular risk factors. This needs a longitudinal study. We did single measurements of the laboratory parameters that could have affected accuracy of the results. The study enrolled only those patients present at the clinic during the study period and this could have caused a selection bias. We did not screen our patients for HIV and this could have affected our results.
Conclusion
The present study showed that AIP was significantly elevated and correlated significantly with waist-hip ratio and atherocardiovascular indices among T2D patients in Uganda. AIP at no added cost can be used to stratify patients in a poor resource setting so that those at high risk can be started on primary CVD management with appropriate medications and life style programs.
Acknowledment
Our appreciation goes to Professor Moffat of Medical Research Council Uganda, Mengo Hospital, Star Pharmaceuticals, and Wide Spectrum for funding the study. We are also grateful to the study coordinator, study nurses, study participants, and medical superintendents of the study health facilities.
Conflict of Interest
None.
Authors' contributions
William Lumu, Silver Bahendeka, Ronald Wesonga, and Davis Kibirige, conceptualized and designed the study design. Ronald Wesonga developed the statistical plan for the study. William Lumu was the Principal Investigator of this study. Ronald Mutebi and Emmanuel Ssendikwanawa analysed data with the guidance of William Lumu. Silver Bahendeka was the supervisor of the study. William Lumu, Silver Bahendeka and Ronald Wesonga and Davis Kibirige contributed to the writing of the manuscript. All the authors revised and approved the final version.
References
- 1.Low Wang Cecilia C, Hess Connie N, Hiatt William R, Goldfine Allison B. Clinical Update: Cardiovascular Disease in Diabetes Mellitus. Circulation. 2016;133(24):2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vergès Bruno. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58(5):886–899. doi: 10.1007/s00125-015-3525-8. 2015/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casaccia Bertoluci Marcello, Zorzanelli Rocha Viviane. Cardiovascular risk assessment in patients with diabetes. Diabetology and Metabolic Syndrome. 2017;9(1):1–13. doi: 10.1186/s13098-017-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HAFFNER STEVEN M, LEHTO SEPPO, RÖNNEMAA TAPANI, KALEVI PYÖRÄLÄ, LAAKSO MARKKU. Mortality From Coronary Heart Disease in Subjects with and Without T Ype 2 Diabetes Mortality from Coronary Heart Disease in Subjects with Type 2 Diabetes and in Nondiabetic Subjects with and Without Prior Myocardial Infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 5.Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis: Original Article: Epidemiology. Diabetic Medicine. 2009;26(2):142–148. doi: 10.1111/j.1464-5491.2008.02640.x. [DOI] [PubMed] [Google Scholar]
- 6.Rana Jamal S, Liu Jennifer Y, Moffet Howard H, Jaffe Marc, Karter Andrew J. Diabetes and Prior Coronary Heart Disease are Not Necessarily Risk Equivalent for Future Coronary Heart Disease Events. Journal of General Internal Medicine. 2016;31(4):387–393. doi: 10.1007/s11606-015-3556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg Ira J. Diabetic Dyslipidemia: Causes and Consequences. The Journal of Clinical Endocrinology & Metabolism. 2001;86(3):965–971. doi: 10.1210/jcem.86.3.7304. [DOI] [PubMed] [Google Scholar]
- 8.Harini Nimmanapalli, Ambika Kasi, Prabath Devapatla, Nutakki Vani. Lipid ratios, atherogenic coefficient and atherogenic index of plasma as parameters in assessing cardiovascular risk in type 2 diabetes mellitus. International Journal of Research in Medical Sciences. 2016:2863–2869. [Google Scholar]
- 9.Abd Al-Sattar Dalal, Zaid Naji Hassan1 Asaad1, Shalash Sultan1 Ali. Atherogenic Index of Plasma among Type2 Diabetic Patients Cross-Sectional Study in Iraq. Medico Legal Update. 2020;20(1 SE-Articles):560–564. [Google Scholar]
- 10.Millan Jesus, Pinto Xavier, Munoz Anna, Zuniga Manuel, Rubies-Prat Joan, Pallardo Luis Felipe, et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vascular health and risk management. 2009;5:757–765. [PMC free article] [PubMed] [Google Scholar]
- 11.Bhardwaj Shilpa, Pharm Int J, Sci Bio. Bhardwaj S, Bhattacharjee J, Bhatnagar MK, et al. Atherogenic Index of Plasma, Castelli Risk Index and Atherogenic Coefficient-New Parameters in Assessing Cardiovascular Risk. International Journal of Pharmacy and Biological Sciences. 2013;3(3):359–364. [Google Scholar]
- 12.Zhu Xiaowei, Yu Lugang, Zhou Hui, Ma Qinhua, Zhou Xiaohua, Lei Ting, et al. Atherogenic index of plasma is a novel and better biomarker associated with obesity: a population-based cross-sectional study in China. Lipids in Health and Disease. 2018;17(1) doi: 10.1186/s12944-018-0686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Committee American Diabetes Association Professional Practice, author. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2021;45(Supplement_1):S144–S174. doi: 10.2337/dc22-S010. [DOI] [PubMed] [Google Scholar]
- 14.Kibirige Davis, Atuhe David, Kampiire Leaticia, Kiggundu Daniel Ssekikubo, Donggo Pamela, Nabbaale Juliet, et al. Access to medicines and diagnostic tests integral in the management of diabetes mellitus and cardiovascular diseases in Uganda: insights from the ACCODAD study. International Journal for Equity in Health. 2017;16(1):154. doi: 10.1186/s12939-017-0651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olamoyegun MA, Oluyombo R, Asaolu SO. Evaluation of dyslipidemia, lipid ratios, and atherogenic index as cardiovascular risk factors among semi-urban dwellers in Nigeria. Annals of African medicine. 2016;15(4):194–199. doi: 10.4103/1596-3519.194280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.William Lumu. Atherosclerotic Cardiovascular Disease Risk Among Patients with Type 2 Diabetes in Uganda. Texila International Journal of Academic Research. 2021;8(2):51–66. [Google Scholar]
- 17.Bolarinwa A, Ahmed A, Bello A, Obalowu I, Sani M, Biliaminu S, et al. Atherogenic risk pattern among Nigerian hypertensive and diabetic patients undergoing follow-up visit in a tertiary hospital. Research Journal of Health Sciences. 2018;6:208–217. [Google Scholar]
- 18.Ross Robert, Neeland Ian J, Yamashita Shizuya, Shai Iris, Seidell Jaap, Magni Paolo, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nature Reviews Endocrinology. 2020;16(3):177–189. doi: 10.1038/s41574-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noumegni Steve Raoul, Nansseu Jobert Richie, Bigna Jean Joel, Ama Moor Vicky Jocelyne, Kembe Assah Felix, Dehayem Mesmin Y, et al. Atherogenic index of plasma and 10-year risk of cardiovascular disease in adult Africans living with HIV infection: A cross-sectional study from Yaoundé, Cameroon. JRSM Cardiovascular Disease. 2017;6:2048004017740478. doi: 10.1177/2048004017740478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wekesa Clara, Asiki Gershim, Kasamba Ivan, Waswa Laban, Reynolds Steven J, Nsubuga Rebecca N, et al. Atherogenic Risk Assessment among Persons Living in Rural Uganda. Tanner Marcel, editor. Journal of Tropical Medicine. 2016;2016:7073894. doi: 10.1155/2016/7073894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saz1n Seher, Kutlu R, Ko ak A. The relationship between atherogenic index of plasma and major risk factors of cardiovascular disease in obese and non-obese individuals. The European Research Journal. 2018;5:678–685. PubMed. [Google Scholar]
- 22.Niroumand Shabnam, Khajedaluee Mohammad, Khadem-Rezaiyan Majid, Abrishami Maryam, Juya Mohammadreza, Khodaee Gholamhasan, et al. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Medical journal of the Islamic Republic of Iran. 2015;29:240. [PMC free article] [PubMed] [Google Scholar]
- 23.Bo Myat Su, Cheah Whye Lian, Lwin Soe, Moe Nwe Tin, Win Than Than, Aung Myint. Understanding the Relationship between Atherogenic Index of Plasma and Cardiovascular Disease Risk Factors among Staff of a University in Malaysia. Schiavo Luigi, editor. Journal of Nutrition and Metabolism. 2018;2018:7027624. doi: 10.1155/2018/7027624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmena Rafael, Duriez Patrick, Fruchart Jean Charles. Atherogenic Lipoprotein Particles in Atherosclerosis. Circulation. 2004;109(23_ PubMed suppl_1) doi: 10.1161/01.CIR.0000131511.50734.44. [DOI] [PubMed] [Google Scholar]
- 25.Cai Gaojun, Shi Ganwei, Xue Sheliang, Lu Wei. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine. 2017;96(37) doi: 10.1097/MD.0000000000008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millán Jesús, Pintó Xavier, Muñoz Anna, Zúñiga Manuel, Rubiés-Prat Joan, Pallardo Luis Felipe, et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Circulation. 2017;7(1):13–27. [PMC free article] [PubMed] [Google Scholar]
- 27.Choudhary MK, Eräranta A, Koskela J, Tikkakoski AJ, Nevalainen PI, Kähönen M, et al. Atherogenic index of plasma is related to arterial stiffness but not to blood pressure in normotensive and never-treated hypertensive subjects. Blood Pressure. 2019;28(3):157–167. doi: 10.1080/08037051.2019.1583060. [DOI] [PubMed] [Google Scholar]
- 28.Meigs James B, Singer Daniel E, Sullivan Lisa M, Dukes Kimberly A, D'Agostino Ralph B, Nathan David M, et al. Metabolic Control and Prevalent Cardiovascular Disease in Non-Insulin-dependent Diabetes Mellitus (NIDDM): The NIDDM Patient Outcomes Research Team. American Journal of Medicine, The. 1997;3526(1) doi: 10.1016/s0002-9343(96)00383-x. 7- YP – 132. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Hongmei, Qin Li, Sheng Chang Sheng, Niu Yixin, Gu Hongxia, Lu Shuai, et al. ASCVD risk stratification modifies the effect of HbA1c on cardiovascular events among patients with type 2 diabetes mellitus with basic to moderate risk. BMJ Open Diabetes Research & Care. 2020;8(1):e000810. doi: 10.1136/bmjdrc-2019-000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavero-Redondo Iván, Peleteiro Barbara, Álvarez-Bueno Celia, Rodriguez-Artalejo Fernando, Martínez-Vizcaíno Vicente. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis. BMJ Open. 2017;7(7):e015949. doi: 10.1136/bmjopen-2017-015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mannucci Edoardo, Dicembrini Ilaria, Lauria Angelo, Pozzilli Paolo. Is Glucose Control Important for Prevention of Cardiovascular Disease in Diabetes? Diabetes Care. 2013;36(Supplement 2) doi: 10.2337/dcS13-2018. S259 LP-S263. [DOI] [PMC free article] [PubMed] [Google Scholar]