Figure 1.
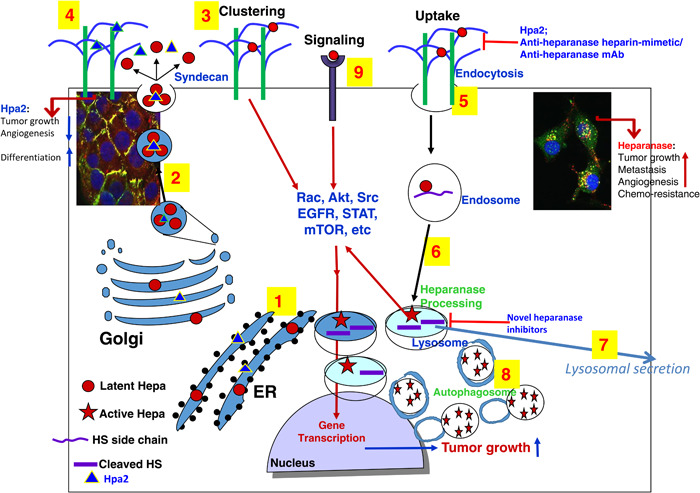
Schematic presentation of heparanase and Hpa2 biosynthesis and trafficking. Pre–proheparanase (red circles) and Hpa2 (blue triangles) are first targeted to the ER lumen via their own signal peptides (1). The proteins are then shuttled to the Golgi apparatus and are subsequently secreted via vesicles that bud from the Golgi (2). Once secreted, heparanase rapidly interacts with syndecans, resulting in their clustering and signaling (3), followed by rapid endocytosis of the heparanase–syndecan complex (5) that accumulates in late endosomes (6). Hpa2 interacts with cell membrane HSPG (i.e., syndecans) with higher affinity but unlike heparanase, is not subjected to uptake but rather remains on the cell membrane for a relatively long period of time (4 and left inset). Accumulation of Hpa2 in the extracellular compartment is enhanced by heparin or anti‐Hpa2 monoclonal antibody. Heparanase uptake is inhibited by heparin/heparin mimetics, antiheparanase monoclonal antibodies, or Hpa2, resulting in extracellular accumulation of the latent enzyme (5). Conversion of endosomes to lysosomes (6) results in heparanase processing and activation (primarily by cathepsin L) awaiting secretion (7). Typically, heparanase appears in perinuclear lysosomes (right inset), promoting autophagy (8) and tumor growth, metastasis, angiogenesis, and chemoresistance due to its enzymatic and signaling (9) functions. Hpa2, on the other hand, attenuates tumor growth and vascularity. Novel heparanase inhibitors are expected to target extracellular latent (signaling) and active heparanase as well as the intracellular, lysosomal, enzyme. ER, endoplasmic reticulum; Hpa2, heparanase‐2; HSPG, heparan sulfate proteoglycan.
