Figure 5. RIF1 binds SHLD3 through polar residues within its extreme N terminus.
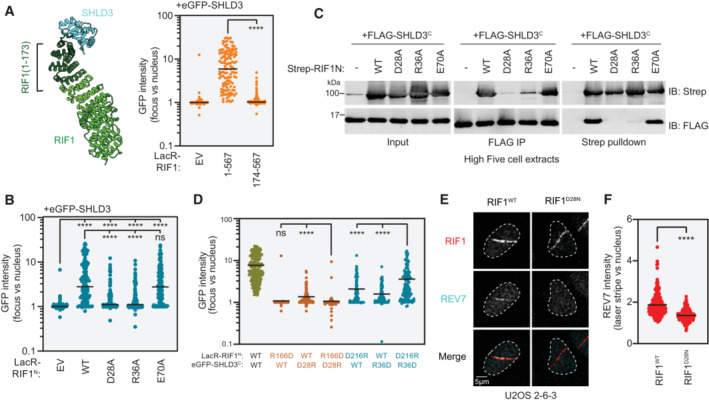
- Left: Representation of the region of the RIF1 N‐terminal HEAT repeats that are predicted to bind the SHLD3 eIF4E‐like domain. Residues 1–173 are highlighted. Right: Quantification of LacR/LacO assay measuring eGFP‐SHLD3 recruitment to LacO arrays in U2OS 2‐6‐3 cells by the indicated truncated mCherry‐LacR‐RIF1N variants. GFP intensities are presented as a ratio between the average fluorescence intensity within the mCherry‐labeled LacR‐RIF1N focus and the average nuclear intensity. Bars represent mean values (n = 148, 123, 122 for EV, 1–567, 174–567 from three biologically independent experiments). Analysis was performed using the Mann–Whitney two‐tailed test. ****P < 0.001. See also Fig EV4A and B. EV—empty vector.
- Quantification of LacR/LacO assay measuring eGFP‐SHLD3 recruitment to LacO arrays in U2OS 2‐6‐3 cells by mCherry‐LacR‐RIF1N (residues 1–967) or the indicated alanine substitution variants. GFP intensities are presented as a ratio between the average fluorescence intensity within the mCherry‐labeled LacR focus and the average nuclear intensity. Bars represent mean values (n = 142, 121, 132, 135, 127 for EV, WT, D28A, R36A, E70A from three biologically independent experiments). Analysis was performed using the Kruskal–Wallis test followed by Dunn's multiple comparisons against empty vector (EV) and wild‐type RIF1N (WT) controls. ****P < 0.001, ns P > 0.05. See also Fig EV4C and D.
- Whole cell extracts of High Five insect cells individually expressing the FLAG‐SHLD3C (residues 126–250) and the indicated alanine substitution Strep‐RIF1 (residues 1–980) variants through baculovirus infection were combined and subjected to FLAG immunoprecipitation or streptactin pulldown and immunoblotted for the Strep or FLAG epitopes. The results are representative of two biologically independent experiments. IB—immunoblot. IP—immunoprecipitation.
- Quantification of LacR/LacO assay measuring eGFP‐SHLD3C recruitment to LacO arrays in U2OS 2‐6‐3 cells by mCherry‐LacR‐RIF1N with both transfected plasmids bearing charge‐reversal mutations. Bars represent mean values (n = 222, 128, 121, 135, 131, 127, 128 for WT/WT, R166D/WT, WT/D28R, R166D/D28R, D216R/WT, WT/R36D, D216R/R36D from five biologically independent experiments). Analysis was performed using the Kruskal–Wallis test followed by Dunn's multiple comparisons. ****P < 0.0001, ns P > 0.05. See also Fig EV4E and F.
- Representative micrographs of UV laser microirradiation experiments measuring DNA‐damage recruitment of REV7 in U2OS 2‐6‐3 cells with endogenously mutated RIF1. DNA damage was induced in U2OS 2‐6‐3 cells through irradiation in the form of linear stripes and analyzed by immunofluorescence microscopy with RIF1 and REV7 antibodies. See also Fig EV4G and H.
- Quantification of UV laser microirradiation immunofluorescence experiment measuring DNA‐damage recruitment of REV7 in U2OS 2‐6‐3 cells with endogenously mutated RIF1. REV7 immunofluorescence intensities are presented as a ratio between the average fluorescence intensity within the RIF1‐labeled irradiation stripe and the average nuclear intensity. Only nuclei containing RIF1 stripes are quantified. Bars represent mean values (n = 206, 215 for RIF1WT, RIF1D28N from two biologically independent experiments). Analysis was performed using the Welch's t‐test. ****P < 0.0001.
Source data are available online for this figure.
