Figure 6.
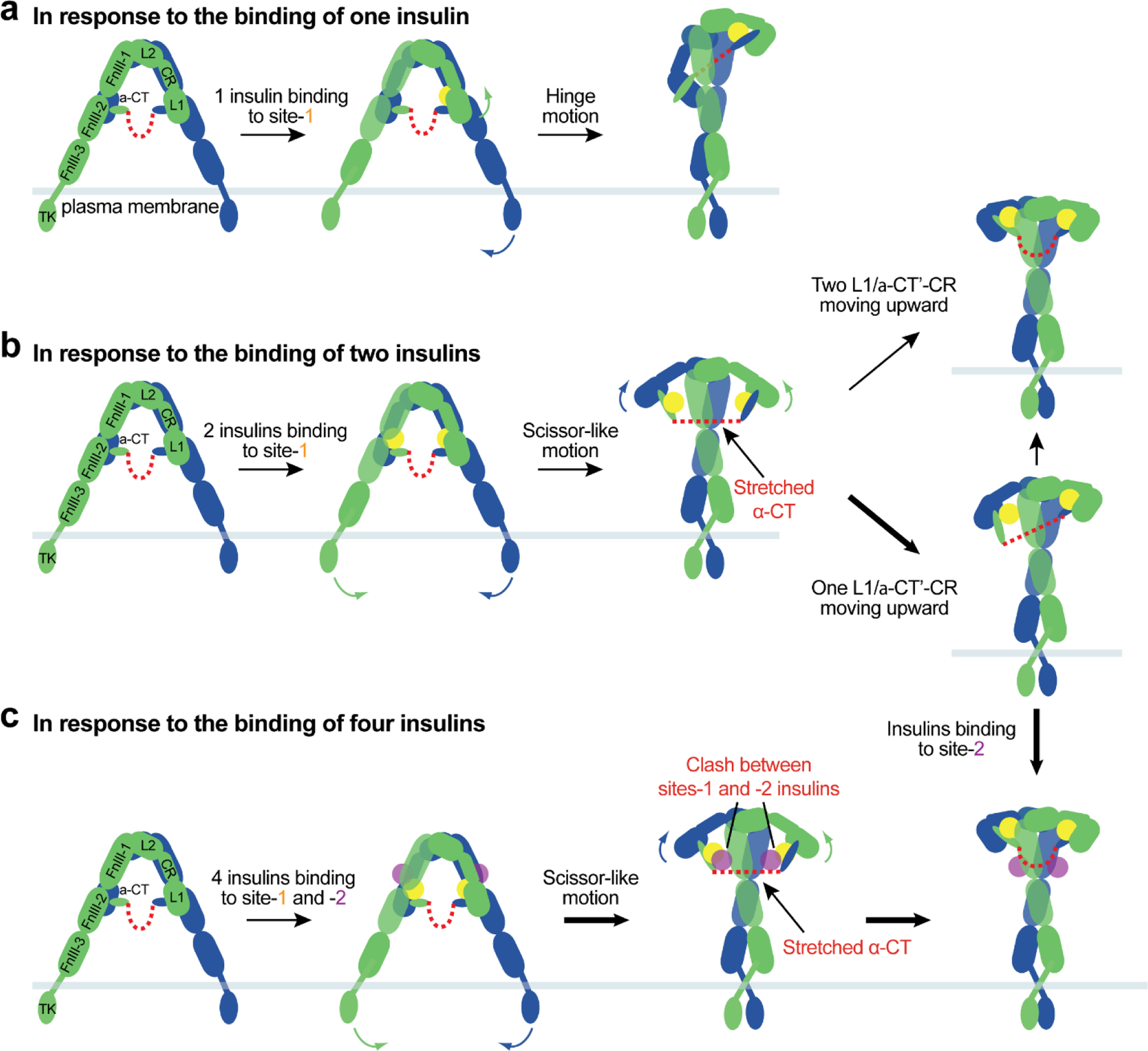
The proposed model of insulin-induced IR activation. At unsaturated insulin concentrations, one or two insulins bind to IR site-1(s) and disrupt the auto-inhibited conformation. (a) The released L1/α-CT’ domains with a site-1a insulin move upward to the top-loop of FnIII-1’ (site-1b), crosslinking two protomers. (b) Two insulins binding at both IR site-1s facilitates a scissor-like rotation of two protomers. We speculate that these conformational changes lead to an instable, intermediate state of IR. We further propose that hinge motions between the L1/α-CT’-CR-L2 domains within one or two protomers reduce the structural instability, resulting in ‘Ƭ’-shaped asymmetric or ‘T’-shaped symmetric IR dimers. (c) At saturating insulin concentrations, four insulins bind to IR sites-1 and −2, breaking the auto-inhibited conformation effectively and promoting a scissor-like rotation of two protomers. The collision between two insulins at sites-1 and −2 in combination with the tension generated by disulfide-linked α-CTs would promote the formation of the fully active, ‘T’-shaped IR.
