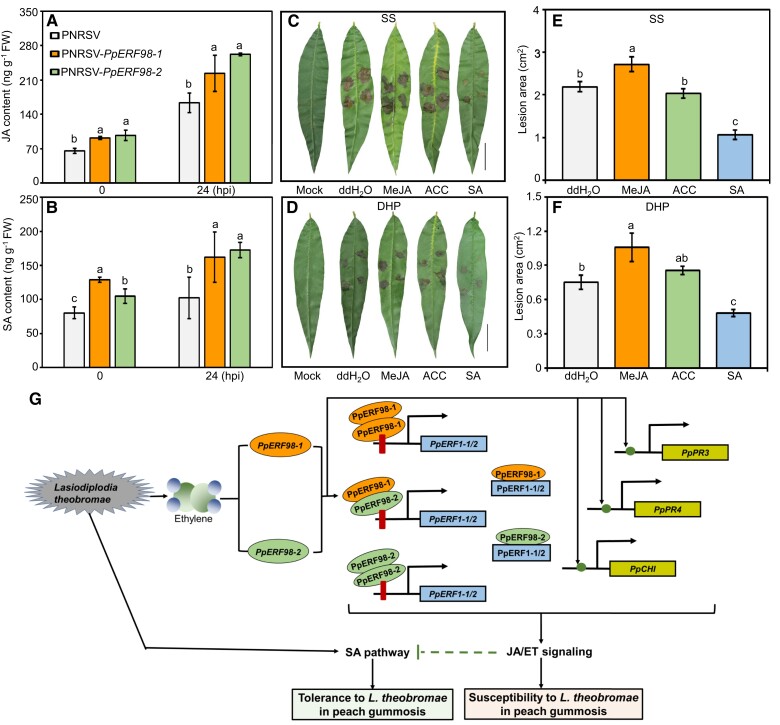
Figure 7.
The defense pathway of JA/ET and SA are regulated by PpERF98-1/2. A and B) Accumulation of JA and SA in the PpERF98-1/2-silenced peach seedlings at 0 and 24 hpi with L. theobromae. C to F) Lesion symptom and size in the hormone-treated leaves of the susceptible cultivar SS and the tolerant cultivar DHP prior to L. theobromae inoculation. Fully expanded leaves were evenly sprayed with 100 µM MeJA, 500 µM SA, 500 µM ACC (ET precursor, 1-aminocyclopropane-1-carboxylate), or ddH2O for 24 h prior to L. theobromae or mock inoculation. Leaf images were digitally extracted with a same scale in panes C) and D), bar = 2 cm. Different letters on top of bars indicate statistical significance at the same time point and P < 0.05 based on Duncan's post hoc test. G) Proposed regulatory model for the role of PpERF98-1 and 2 in peach response to the gummosis fungus L. theobromae. When plants are infected by L. theobromae, large amounts of ET are produced and induce the transcription of PpERF98-1/2. These 2 PpERF98 proteins form heterodimers and homodimers with themselves and directly bind to the GCC-box cis-elements of the promoters of PpERF1-1/2, activating their transcription. Further, these 2 PpERF98 proteins interact with 2 PpERF1 proteins to form heterodimers and amplify JA/ET signaling. Moreover, the PpERF98s can also activate the transcription of the JA/ET-responsive genes PpPR3, PpPR4, and PpCHI through putative ERF-binding cis-elements. Blocking the JA and ET pathways can decrease peach susceptibility to L. theobromae, while SA can promote tolerance (our earlier work; Zhang et al. 2022). Altogether, our data show that 2 interacting PpERF98 genes negatively regulate peach resistance to L. theobromae by activating JA/ET signaling to attenuate the SA-dependent defense pathway. Red bars represent GCC-box, and solid green circles indicate other putative ERF-binding sites in target genes.