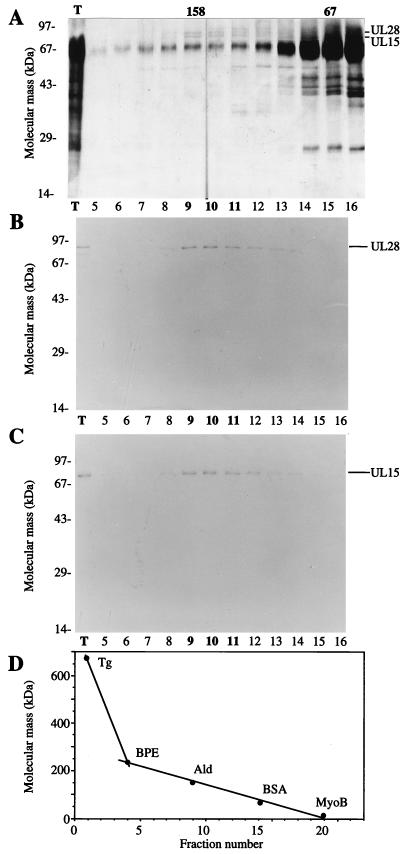
FIG. 3.
Copurification of UL15 and UL28 from infected cell extracts. Proteins purified from HSV(KOS)-infected nuclear extracts were loaded onto the final sucrose gradient, and the indicated aliquots were separated by SDS-PAGE. Proteins were detected by silver staining (A), Western blotting with anti-UL28 H85 antiserum (B), or Western blotting with anti-UL15 AS7 antiserum (C). An aliquot of the total protein sample (T) loaded onto the sucrose gradient is indicated (A to C). Western blot analyses of proteins on nitrocellulose membranes included alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (Boehringer-Mannheim) and the colorimetric conversion of 5-bromo-4-chloro-3-indolylphosphate (BCIP)-nitroblue tetrazolium (NBT) (Kirkegaard and Perry Laboratories). The peak fractions of UL15 and UL28 are indicated in bold type. (D) Sedimentation size standards were run in a parallel gradient: thyroglobulin (Tg, 670 kDa), β-phycoerythrin (BPE, 240 kDa), aldolase (Ald, 158 kDa), BSA (67 kDa), and myoglobin (MyoB, 17 kDa). The 158- and 67-kDa markers correspond to the peak fractions of aldolase and BSA.