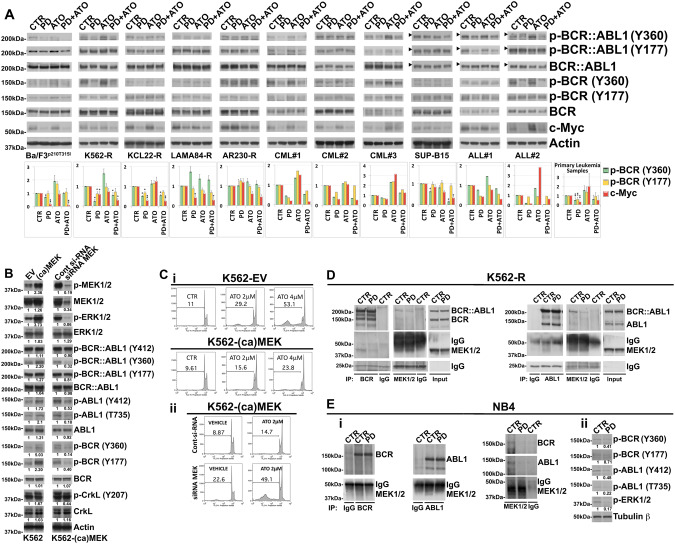
Fig. 4. MEK1/2 functionally interact with normal BCR and ABL1 in TKI-resistant Ph+ leukemia cells.
A Analysis of endogenous phospho-BCR::ABL1 (Tyr360) and (Tyr177), BCR::ABL1, phospho-BCR (Tyr360) and (Tyr177), BCR, c-Myc and Actin as loading control in murine Ba/F3p210T315I cells, Imatinib-resistant Ph+ leukemia cell lines and primary cells from Ph+ leukemia patients following 24 h of sequential and combined treatment with PD0325901 (0.5 μM) and ATO (2 μM). The horizontal arrowheads indicate the position of the 200 kDa protein marker for Ph+ ALL cell line and primary blasts from Ph+ ALL patients. The graphs show the relative fold change of phospho-BCR (Tyr360) and (Tyr177) normalized to total BCR and c-Myc normalized to Actin expression. Phospho-BCR::ABL1 (Tyr360) and phospho-BCR (Tyr360), Phospho-BCR::ABL1 (Tyr177) and phospho-BCR (Tyr177), BCR::ABL1 and BCR are from the same blot. Phosphorylation status of BCR and c-Myc expression under control conditions were set as 1 for comparison and are shown in the histograms (mean ± SD of three independent blots of Ph+ cell lines and of n = 5 primary samples; °p < 0.05, *p < 0.01, **p < 0.005, vs. untreated control cells; Dunnett test). B K562 cells expressing empty vector control (EV) or constitutive-active form of MEK1 (ca-MEK) were subjected to Western Blot analysis to monitor the expression of phospho-MEK1/2 (Ser217/221), MEK1/2, phospho-ERK1/2 (Thr202/Tyr204), ERK1/2, phospho-BCR::ABL1 (Tyr412), phospho-BCR::ABL1 (Tyr360), phospho-BCR::ABL1 (Tyr177), BCR::ABL1, phospho-ABL1 (Tyr412), phospho-ABL1 (Thr735), ABL1, phospho-BCR (Tyr360) and (Tyr177), BCR, phospho-CrkL (Tyr207), CrkL and Actin. K562-(ca)MEK cells were then electroporated with control siRNA or with MEK1/2 siRNA and after 24 h total cell lysates were subjected to western blot analysis of the same panel of proteins described above in panel. C (i) Relative levels of apoptosis revealed in stable clones of K562 cells transfected with empty vector (EV) or with plasmid expressing Activated MEK1 [(ca)MEK] after 72 h of ATO (2 and 4 μM) treatment. (ii) Relative levels of apoptotic cell death in K562-(ca)MEK cells subjected to the ATO (2 μM) treatments following siRNA knockdown of MEK1/2 or transfection with a non-targeting control siRNA (Cont), as determined by flow cytometric analysis of sub-G1 DNA content. D K562-R cell line was treated with PD0325901 (0.5 μM) and after 24 h of treatment subjected to immunoprecipitation (IP) using anti-BCR antibody or anti-MEK 1/2 antibody or control antibody (IgG) and immunoblotted with anti-BCR and anti-MEK1/2 antibodies. In the same way K562-R lysates were subjected also to immunoprecipitation (IP) using anti-ABL1 antibody or anti-MEK 1/2 antibody or control antibody (IgG) and immunoblotted with anti-ABL1 and anti-MEK1/2 antibodies. For comparison, cell lysates from K562-R cells were loaded in the same gel. E (i) NB4 acute leukemia cell line was treated with PD0325901 (0.5 μM) and after 24 h subjected to immunoprecipitation (IP) using anti-BCR antibody or anti-ABL1 antibody, anti-MEK 1/2 antibody or control antibody (IgG) and immunoblotted against BCR, ABL1 and MEK1/2. (ii) The same cell lysates were immunoblotted against phospho-BCR (Tyr360), phospho-BCR (Tyr177), phospho-ABL1 (Tyr412), phospho-ABL1 (Thr735), phospho-ERK1/2 (Thr202/Tyr204) and Tubulin β as loading control. Western blot results were subjected to densitometric scanning and normalized to Tubulin β expression. Protein expression under control conditions was set as 1 for comparison and is shown below the blots.