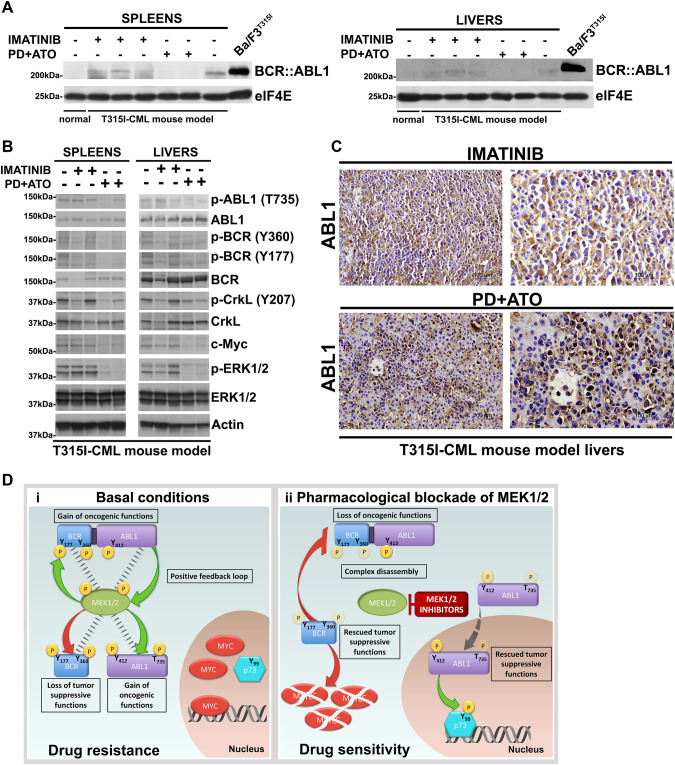
Fig. 7. Combined treatment with MEK1/2 inhibitor PD0325901 and ATO activates the tumor suppressor functions of BCR and ABL1 in vivo.
A Spleens and livers from mice receiving vehicle, Imatinib or PD + ATO treatments were harvested 20 days after leukemia cell inoculation and processed for Western blot analysis to determine the expression of BCR::ABL1 protein and elF4E, used as loading control. Spleens and livers of non-inoculated control mice as well as lysates from Ba/F3p210T315I cells were examined for comparison. B Western blot analysis of phospho-ABL1 (Thr735), ABL1, phospho-BCR (Tyr360), phospho-BCR (Tyr177), BCR, phospho-CrkL (Tyr207), CrkL, c-Myc, phospho-ERK1/2 (Thr202/Tyr204), ERK1/2, and Actin on spleens and livers from mice receiving vehicle, Imatinib or PD + ATO treatments. C Livers explanted from leukemic mice treated with Imatinib or PD + ATO were examined by immunostaining for ABL1. The majority of leukemic cells in the livers of mice treated with PD + ATO show nuclear staining for ABL1. D (i) Under basal conditions MEK1/2 form a complex with and directly phosphorylate BCR, ABL1 and BCR::ABL1 at specific residues thus provoking the loss of BCR’s tumor-suppression functions, cytoplasmic retention of ABL1 with oncogenic functions and an enhanced oncogenic activity of BCR::ABL1. In turn, BCR::ABL1 activates MEK1/2 to create a MEK1/2/BCR::ABL1/ABL1/BCR signaling-centered positive feedback loop that stabilizes MYC and disables p73 pro-apoptotic functions to induce drug resistance. (ii) Pharmacological blockade of MEK1/2 disrupts the MEK1/2/BCR::ABL1/ABL1/BCR kinases complex leading to BCR::ABL1Y177/Y360, BCRY177/Y360 and cytoplasmic ABL1Y412/T735 dephosphorylation and consequently an attenuated oncogenic BCR::ABL1 signaling, the rescue of the BCR kinase anti-oncogenic functions and the nuclear translocation of ABL1, event that converts the tyrosine kinase from a cytoplasmic oncogenic to a nuclear tumor-suppressive effector. These molecular changes render TKI-resistant leukemic cells vulnerable to ABL1 allosteric activators or arsenic trioxide via activation of ABL1-p73 and BCR-MYC axes.