Abstract
Background and Aims
The present study aimed to review probiotics' clinical efficacy in preventing infectious diseases among hospitalized patients in ICU and non‐ICU wards.
Methods
A search of Medline, EMBASE, The Cochrane Library, Science Direct, Open Grey, and Google Scholar was conducted for eligible publications from 2002 to 2020 following the requirements outlined in the PRISMA guideline. The search strategy was based on the combination of the following terms: “probiotics,” “prebiotics,” “synbiotics,” and “cross‐infection.” The logical operators “AND” (or the equivalent operator for the databases) and “OR” (e.g., probiotics OR prebiotics OR synbiotics) were used.
Results
The results indicated that the probiotic consumption caused a significant reduction in antibiotic‐associated diarrhea (AAD) and Clostridioides difficile infection (CDI) in 2/8 randomized clinical trials (RCTs) investigating AAD/CDI. Also, 5/12 clinical trials highlighted the considerable effects of probiotics on the reduction or prevention of ventilator associated pneumoniae (VAP), so the mean prevalence of VAP was lower in the probiotic group than in the placebo group. The total rate of nosocomial infections among preterm infants was nonsignificantly higher in the probiotic group compared to the control group.
Conclusion
This systematic review shows that the administration of probiotics has moderate preventive or mitigating effects on the occurrence of VAP in ICU patients, CDI, AAD, and nosocomial infections among children. Consequently, applying antibiotics along with the proper probiotic species can be advantageous.
Keywords: antibiotic‐associated diarrhea, Clostridioides difficile infection, nosocomial infections, probiotic, ventilator‐associated pneumonia
1. INTRODUCTION
According to the International Scientific Association, probiotics and prebiotics are defined as live microorganisms that, when administered in adequate quantities, confer some health benefits to the host. 1 Many probiotics contain mixtures of two or more individual species. Most probiotic regimens include the two genera of Lactobacillus and Bifidobacterium, constituting the central part of the normal intestinal microflora among humans. 2 Probiotic strains exert their antimicrobial properties through the production of ammonia, lactic acid, free fatty chains, hydrogen peroxide, and bacteriocins. Moreover, probiotics affect the intestinal ratio of beneficial and harmful bacteria in favor of the growth of beneficial bacteria. 3 Current evidence from various research indicates that the action mechanism of each probiotic strain might be unique and cannot be analogized by other species. In addition, the effects of each probiotic strain also depend on the ingested regimen quantity, the frequency of intakes and even the disease type for which the strain is being used. 4
Two factors render the application of probiotics in modern therapeutics: limited financial resources for the introduction of novel antibiotics; and a progressive understanding of the role of probiotics in interactions with microbiota for the prevention of infectious diseases. 5 Probiotics have increasingly been recognized to prevent various infectious diseases and restore the digestive flora, which might have changed during various diseases or following antibiotic treatment. 6 Most clinical trials proved the beneficial role of probiotics in the prevention or reduction of some specific infectious disorders, including antibiotic‐associated diarrhoea (AAD) and Clostridioides difficile infection (CDI) among children and adults, acute gastroenteritis in adults, necrotizing enterocolitis (NEC) in neonates and ventilator‐associated pneumonia (VAP) in adults. 7 Hence, due to the many benefits of probiotics, the present study aimed to review the clinical efficacy of probiotics in preventing infectious diseases among hospitalized patients in the ICU.
2. MATERIALS AND METHODS
This systematic review was carried out following the requirements outlined in the PRISMA guideline (Preferred Reporting Items for Systematic Reviews and Meta‐Analysis). 8 This study was approved and supported by the Ethics Committee (IR SHAHED.REC.1399.162) of the Molecular Microbiology Research Center of Shahed University.
2.1. Data sources and research records
A search of Medline (https://www.ncbi.nlm.nih.gov/mesh), EMBASE (https://www.embase.com), The Cochrane Library (https://www.cochranelibrary.com), Science Direct (https://www.sciencedirect.com), Open Grey (http://www.opengrey.eu), and Google Scholar was conducted for eligible publications from 2002 to 2020.
The search strategy was based on the combination of the following terms: “probiotics,” “prebiotics,” “synbiotics,” and “cross‐infection.” The logical operators “AND” (or the equivalent operator for the databases) and “OR” (e.g., probiotics OR prebiotics OR synbiotics) were used to combine all descriptors to improve the results. Moreover, the search strategy was adapted to the particularities of each database. Whenever possible, synonyms were searched, or the option of searching for similar terms was used before every keyword. Bibliographies of the reviews found during our search were also checked to identify any additional relevant studies. Articles deemed potentially eligible were retrieved for a full‐text review.
2.2. Inclusion and exclusion criteria
Figure 1 summarizes the article exploring procedures. Only high‐quality, full‐text, and well‐described randomized controlled trials (RCTs) on adults or children with defined outcomes and published in English were included in this review. Reviews, in vitro studies, nonrandomized trials and case–control studies, duplicate reports, comments, notes, opinion pieces, methodological reports, or conference abstracts were excluded.
Figure 1.

PRISMA flow chart of the study selection procedure.
One reviewer did an initial screening of search results to exclude irrelevant records. Two independent reviewers screened the remaining records to identify the potentially relevant records meeting the inclusion/exclusion criteria based on the title, abstract analysis, and the full text in the second stage. A third reviewer resolved disagreements. After screening, duplicate studies were excluded.
2.3. Data extraction and studies characteristics
Data were extracted separately by two reviewers, and discrepancies were resolved by consensus. The following data of each included study were extracted: (a) publication characteristics (first author, year, country, study design); (b) characteristics of the participants (sample size, patients' age, gender); and (c) probiotics strain, probiotics dosage, intervention, controls used and the duration of therapy; (d) primary outcomes.
3. RESULTS AND DISCUSSION
A total of 2650 articles were retrieved by searching international databases. The summary of the search and studies selection method is shown in the Prisma flow chart (Figure 1). In the second screening phase, 651 publications were excluded based on their title and abstract evaluation, and 156 articles were retained for detailed full‐text evaluation. After full‐text evaluation, 54 articles describing the efficacy of the probiotics on cross‐infection were selected for further analysis.
The outcomes of 54 different clinical trials evaluating the clinical efficacy of probiotics in reducing or preventing infectious diseases are described in Table 1. The participants were males, 53.36% (n = 8896) and 46.64% females (n = 7776), with the age range from newborn infants to 97‐year‐old. Moreover, 8469 (50.80%) participants were included in the probiotic groups and 8203 (49.20%) in the placebo groups. Among the 44 clinical trials, 38 used probiotics, 5 trials used synbiotics and one used prebiotics.
Table 1.
The outcomes of different clinical trials assessing the clinical efficacy of probiotics on the reduction or prevention of infectious diseases.
| Reference | Country | Sample size | Mean age (sd) | Study design | Participant's characteristics | Probiotics | Dose (cfu) | prebiotics | Intervention | Control used | p Value | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [9] | USA | 33 | 65 | RCT | Initial episode of mild to moderate CDI | Lactobacillus acidophilus | 1.7 × 1010 | NR | One capsule/o.d/28 days | NR | <0.05 | Probiotics were introduced as a promising adjunct therapy for the treatment of an initial CDI |
| Lacticaseibacillus paracasei | ||||||||||||
| Bifidobacterium lactis Bi‐07 | ||||||||||||
| B. lactis Bl‐04 | ||||||||||||
| [10] | England | 2981 | 77.2 | RDBPMT | Exposed to one or more oral or IV in the preceding 7 days, or about to start AB treatment | L. acidophilus | 6 × 10¹⁰ | NR | One capsule/b.i.d/21 days |
Inert maltodextrin powder |
0.35 | Probiotics not prevented AAD or CDD |
| Bifidobacterium bifidum | ||||||||||||
| B. lactis | ||||||||||||
| [11] | England | 135 | 73.7 | RDBPCT | Prescribed AB, able to take food and drink orally | Lactobacillus casei | 1.0 × 108 | NR | One hundred grams (97 mL) drink/b.i.d/1 week after the course finished | Sterile milkshake | <0.05 | Probiotics reduced the incidence of AAD and CAD, decreased morbidity and mortality rates, as well as healthcare costs |
| Lactobacillus bulgaricus | 1.0 × 107 | |||||||||||
| Streptococcus thermophilus | 1.0 × 108 | |||||||||||
| [12] | Poland | 250 | 2.1 | RCT | Oral or IV AB therapy, which was started within 24 h of enrolment | Lactobacillus reuteri | 2 × 108 | NR | 2 × 5 drops/b.i.d/the duration of AB treatment for 1 week after AB cessation in drops | NR | NR | Probiotics were ineffective in the prevention of diarrhea or AAD |
| [13] | Italy | 275 | 79.9 ± 9.9 | Single‐center, RDBPCT, parallel‐group | Hospitalized, AB to treat or prevent infectious diseases <48 h | Saccharomyces boulardii | 5 × 10 9 | NR | Capsule/b.i.d/7 days after AB withdrawal, and followed for 12 weeks after ending AB treatment | Rice flour | 0.60 | Probiotics were ineffective in preventing the development of AAD |
| [14] | USA | 302 | 57.2 ± 18.0 | RPCT | IV or oral antibacterial agent | Lactobacillus rhamnosus GG | 20 × 109 | NR | One capsule/b.i.d/14 days | Inulin filler | 0.93 | Probiotics did not reduce the occurrence rate of diarrhea |
| [15] | UK | 229 | 57.9 | DBRPCCT | Taken AB within 4 weeks before admission, high‐risk AB, bowel pathology | Bifidobacterium breve | 450 billion | NR | One sachet/b.i.d/the length of the AB course and for 7 days after that | Maltose and silicon oxide | >0.05 | Probiotics did not reduce the incidence of AAD For CDAD may not be indicated on average‐risk hospital patients |
| Bifidobacterium longum | ||||||||||||
| Bifidobacterium infantis | ||||||||||||
| L. acidophilus | ||||||||||||
| Lactobacillus plantarum | ||||||||||||
| L. paracasei | ||||||||||||
| Lactobacillus delbrueckii subsp. bulgaricus | ||||||||||||
| S. thermophilus | ||||||||||||
| [16] | Canada | 89 | 68.8 ± 14.5 | RDBPCT | Hospitalized, take at least 3 days of any systemic AB | L. acidophilus | 50 × 109 | NR | Lactobacilli‐fermented milk/o.d/21 days | A lactoserum devoid of M. O | <0.05 | Probiotics prevented AAD in hospitalized patients |
| L. casei | ||||||||||||
| [17] | Turkey | 151 | 27.2 ± 8.7 | DBCS | Hospitalized under any systemic AB | S. boulardii | NR | NR | One capsule/b.i.d/during the course of AB therapy | NR | <0.05 | Probiotics reduced AAD in hospitalized patients |
| [18] | Germany | 477 | 58.3 ± 17.15 | RDBPMT | Hospitalized, take any systemic AB | S. boulardii | 1.8 × 1010 | NR | Two‐hundred and fifty milligrams capsules/b.i.d/1 days after AB treatment to 7 days after AB discontion, and followed for 12 weeks after ending AB treatment | NR | >0.05 | There is no evidence for an effect of S. boulardii in preventing AAD or CDAD |
| [19] | China | 255 | 60 ± 6 | Single‐center, RPCDB dose‐ranging study | Hospitalized, take at least 3 days of any systemic AB | L. acidophilus | 50 × 106 | NR | Capsule/b.i.d/36 h after AB treatment to 5 days after AB discontion, and followed for 21 days after ending AB treatment | NR | <0.05 |
Probiotic was effective in reducing the risk of AAD and, in particular, CDAD |
| L. casei | ||||||||||||
| [20] | Bulgaria | 97 | 8.85 ± 3.98 | RDBPCT | Hospitalized children with acute infections | L. reuteri | 1 × 108 | NR | Chewable tablet/o.d/during the entire period of AB treatment to an additional 7 days | NR | >0.05 | There is no evidence of an effect of probiotics in preventing AAD or CDAD |
| [21] | Sweden | 163 | 40.5 ± 7.1 | DBPCS | Hospitalized, take at least 2 days of any systemic AB | L. plantarum | 5 × 107 | NR | Two‐hundred milliliters drink/o.d/48 after AB therapy to the entire period of AB treatment and an additional 7 days | A drink containing blueberries and 5% oats gruel | <0.05 |
Probiotics have a preventive effect on milder gastrointestinal symptoms during treatment with AB |
| [22] | Australia | 70 | 6.55 | Multisite, DBRPCCT | Hospitalized, children with broad‐spectrum oral AB | L. acidophilus | 5.2 × 109 | NR | Probiotic yogurt/o.d/during the entire period of AB therapy |
Pasteurized yogurt containing S. thermophilus and L. bulgaricus |
<0.05 | Probiotic was effective in reducing the risk of AAD in children |
| L. rhamnosus | 8.3 × 109 | |||||||||||
| B. lactis | 5.9 × 109 | |||||||||||
| [23] | South Korea | 214 | 60.5 ± 15.5 | RDBPMT |
Hospitalized, children with respiratory tract infection and consumption of broad‐spectrum oral AB |
L. rhamnosus | 2 × 109 | NR | One capsule/b.i.d/14 days | Maltodextrin | >0.05 | Probiotics did not reduce the rate of occurrence of AAD |
| L. acidophilus | ||||||||||||
| [24] | India | 1127 | 73.6 ± 10.5 | RDBPMT | Hospitalized, take at least 2 days of any systemic AB | L. rhamnosus | NR | Fermentated‐milk, 100 mL/b.i.d/7 days |
Nonfermented acidified |
0.53 | There is no evidence of an effect of probiotics in preventing AAD | |
| L. casei | 1 × 108 | |||||||||||
| L. delbrueckii ssp. Bulgaricus | 1 × 106 | |||||||||||
| [25] | Poland | 240 | 4.5 ± 0.71 | RDBPCT | Hospitalized, children with common infections | S. thermophilus | 1 × 106 | NR |
Capsule/b.i.d/during the entire period of AB treatment |
Nonfat milk and saccharose |
<0.05 | Probiotics reduced the risk of any diarrhea |
| [26] | Canada | 472 | 58.8 ± 18.6 | MU, RPCDB | Hospitalized, take at least 3 days of any systemic AB | L. acidophilus | 5 × 109 | NR | Fermented‐milk/o.d/21 days | Lactoserum |
<0.05 <0.05 |
Probiotic was effective for preventing and reducing the severity of AAD |
| L. casei | ||||||||||||
| [27] | China | 333 | 49.25 ± 35 | Open, RCCT | Hospitalized, with respiratory tract infection take any systemic AB | S. boulardii | NR | NR | Capsule/b.i.d/14 days | NR | S. boulardii appeared to be effective in the prevention of AAD | |
| [28] | China | 503 | 49.93 ± 11.3 | RDRS | Hospitalized, take any systemic AB | L. acidophilus | 4.17 × 109 | NR | One capsule/o.d/the entire period of AB to 7 days additional | <0.05 | Probiotics appeared to lower the risk of AAD and CDAD | |
| L. paracasei | ||||||||||||
| B. lactis | ||||||||||||
| [29] | Iran | 120 | 59.1 ± 12.9 | PRO, DBRCT | ICU, IMV > 48 h | L. casei | 1010 | NR | One capsule/b.i.d/14 days | Sterile maize starch powder | <0.05 | The duration of ICU and hospital stay was also lower in the probiotic group |
| L. acidophilus | ||||||||||||
| L. rhamnosus | ||||||||||||
| L. bulgaricus | ||||||||||||
| B. breve | ||||||||||||
| B. longum | ||||||||||||
| S. thermophilus | ||||||||||||
| [30] | Thailand | 150 | 73.09 ± 13.16 | RCT | IMV > 72 h | L. casei (Shirota strain) | 8 × 109 | NR | Eighty milliliter of L. casei and 80 mL of the aforementioned fermented dairy product/o.d/28 days or after endotracheal tubes removed | Not receive any additional products | >0.05 | The incidence rates of VAP did not reduce |
| [31] | Japan | 77 | 74 | RCT | IMV 3 days after admission to the ICU, having sepsis | B. breve strain Yakult | 108 | galactooligosaccharides | Three grams Yakult BL Seichoyaku and 10 g galactooligosaccharides/o.d/oral intake was initiated | NR | <0.05 | Probiotics modulated the gut microbiota and environment and had preventive effects on the incidence of enteritis and VAP in patients with sepsis |
| L. casei strain Shirota | 108 | |||||||||||
| [32] | China | 235 | 50.2 ± 18.2 | RCMT | IMV ≥ 48 h | Bacillus subtilis | 4.5 × 109 | NR | One capsule/t.i.d/14 days |
Standard preventive strategies |
>0.05 | The incidence of clinically diagnosed VAP was not significant, and incidence of microbiologically confirmed VAP was significant |
| Enterococcus faecalis | 0.5 × 109 | |||||||||||
| [33] | India | 150 | 2.9 ± 3.41 | OLRCT | PICU, IMV > 48 h | L. acidophilus | 3.3 billion | NR | One capsule/b.i.d/7 days discharge | Not receive any placebo | <0.001 | Probiotics reduced the incidence of VAP |
| B. longum | ||||||||||||
| L. rhamnosus | ||||||||||||
| L. plantarum | ||||||||||||
| L. casei | ||||||||||||
| L. bulgaricus | ||||||||||||
| B. infantis | ||||||||||||
| B. breve | ||||||||||||
| S. thermophilus | ||||||||||||
| [34] | Sweden | 150 | 66 | MU, PRO, RCOT | IMV > 24 h | L. plantarum | 1010 | NR | Emulsion/after tracheal extubation or discharge from the ICU | Standard 0.1% CHX solution | >0.05 | Probiotics did not inhibit the colonization of oropharynx and trachea with potentially pathogenic enteric bacteria |
| [35] | Nebraska | 146 | 52.5 ± 19.3 | PRO, RDBPCT | IMV with an endotracheal tube for >72 h | L. rhamnosus GG | 2 × 109 | NR | One capsule/b.i.d/extubation, tracheostomy placement, or death | Inert plant starch inulin | <0.05 | Probiotics prevented VAP |
| [36] | Greece | 72 | 52.9 | RDBPMT |
Severe multiple organ injuries, tracheal intubation, ventilation, ICU |
Pediococcus pentosaceus 5–33:3 | 1011 (each of LABs) | Inulin, beta‐glucan, pectin, and resistant starch as bioactive fibres | Sachet/o.d/15 days | NR | <0.05 | Decrease the risk for sepsis by bloodstream infections and the occurrence of VAP by A. baumannii |
| Leuconostoc mesenteroides 32–77:1 | ||||||||||||
| L. plantarum 2362 | ||||||||||||
| L. paracasei ssp. 19 | ||||||||||||
| [37] | France | 167 | 60.7 ± 15.8 | DBCRPCT | IMV > 2 days | L. rhamnosus GG | 2 × 1010 | NR | One capsule/o.d/until successful weaning | Excipient | >0.05 | The daily prophylactic administration of probiotics cannot be encouraged in the critically ill patient |
| L. casei | ||||||||||||
| L. acidophilus | ||||||||||||
| B. bifidum | ||||||||||||
| [38] | UK | 259 | 49.5 ± 19.6 | PRO, RDBPCT | Enterally fed patients, IMV for 48 h | P. pentosaceus | 1010 | NR | Sachet/b.i.d/at least 2 days |
Cellulose‐based placebo |
>0.05 | Probiotics did not impact on the incidence of VAP in critically ill patients |
| L. mesenteroides | ||||||||||||
| L. paracasei ssp. paracasei | ||||||||||||
| L. plantarum | ||||||||||||
| [39] | China | 52 | 40.5 ± 13.0 | PRO, RPS | Closed head injury; admission within 24 h after trauma; a GCSs between 5 and 8; able to be fed via nasogastric tube within 48 h after admission | B. longum | 109 | NR | Seven sachets/t.t.d/21 days | NR | >0.05 | Probiotics attenuated the deviated Th1/Th2 response induced by severe TBI and decreased the HCAI rate, especially in the late period. Probiotics did not affect ventilator‐associated pneumonia |
| L. bulgaricus | ||||||||||||
| S. thermophilus | ||||||||||||
| [40] | Canada | 1318 | 60.1 ± 16.2 | RPCT | IMV ≥ 72 h | L. rhamnosus GG | 1 × 1010 | NR |
b.i.d/60 days or until discharge from the ICU or until Lactobacillus species was isolated from a sterile site or cultured as the sole or predominant organism from a nonsterile site |
Microcrystalline cellulose | >0.05 | Probiotics did not affect critically ill patients |
| [41] | Italy | 585 |
Ges, 30.8 ± 2.4 (weeks) for NEC 20 ± 7.5 (days) for UTIs 40.3B37.3 (days) Bacterial sepsis 14 (4.7) (days) |
MDBPS | Newborn infants with a gestational age <33 weeks or birth weight <1500 g admitted to NICUs | L. rhamnosus GG | 6 × 109 | NR | Standard milk with L. rhamnosus/o.d/discharge | Maltodextrins | Not significant | Probiotics were ineffective in reducing the incidence of UTIs, NEC and sepsis |
| [42] | Germany | 183 | NR | RCT | VLBW infants <30 weeks of gestation | B. lactis | 2 × 109 | NR | Lyophilized powder mixed with a standard preterm infant human milk fortifier/s.t.d/first 6 weeks of life | NR | 0.9 | Probiotics did not reduce the incidence density of HCAI |
| [43] | USA | 750 | 32 weeks | DBPCT | NICU, birth weight ≤2000 g, hemodynamically stable, ≤48 h of age | L. reuteri | 108 | NR | Five drops of an oil‐based suspension/o.d/death or discharge from the NICU | Oil base | >0.05 | Probiotics did not decrease the rate of the composite outcome and had a protective role. Probiotics also reduced the feeding intolerance and duration of hospitalization |
| [44] | Netherlands | 113 | 32.1 ± 4.6 | RDBPCT |
Preterm infants, gestational age 32 weeks, birth weight 1500 g |
NR | NR | Eighty percent SCGOS/LCFOS and 20% AOS | Increasing doses between days 3 and 30 of life to a maximum of 1.5 g kg−1 d−1 to breast milk or preterm formula | maltodextrin | >0.05 | Prebiotics does not reduce the risk of morbidity related to severe infections in preterm infants |
| [45] | China | 100 | 38.8 ± 1.1 months | DBRCT | NICU, gestational ages of 37–42 weeks | L. casei | 30 billion | NR | One tablet/t.i.d/8 days | Glucose liquid without probiotics | <0.05 | Supplements of probiotics to critically ill neonates enhance immune activity, decrease the occurrence of nosocomial pneumonia and MODS, and reduce days in the hospital |
| L. acidophilus | ||||||||||||
| B. subtilis | ||||||||||||
| E. faecalis | ||||||||||||
| [46] | Poland | 184 | 14.8 ± 9.0 months | MU, RDBPCT | Children, 1–48 months, hospitalization for reasons other than diarrhea | L. reuteri | 1 × 109 | NR |
Duration of hospitalization |
Maltodextrin | >0.05 | L. reuteri was not effective in preventing nosocomial diarrhea in children |
| [47] | Poland | 81 | 11.6 ± 8.7 | DBPCS | Children, 1–3 months, hospitalization for reasons other than diarrhea | L. rhamnosus GG | 6 × 109 | NR | Sachet/b.i.d/duration hospital stay | NR | <0.05 | LGG reduced the risk of nosocomial diarrhea in infants, particularly nosocomial rotavirus gastroenteritis |
| [48] | China | 215 | 12.22 ± 2.87 days | RCT | Newborns with nosocomial enteric infection | E. faecalis | 1.0 × 107 | NR | ½ capsule/b.i.d/discharge | NR | 0.004 |
Probiotics can effectively decrease the occurrence of infections |
| B. longum | ||||||||||||
| L. acidophilus | ||||||||||||
| [49] | USA | 61 | NR | RDBPCT | Pediatric in ICU | L. rhamnosus GG | 10 × 109 | NR | One capsule/o.d/discharge from the hospital, parental request to withdraw from the study, or the death of the patient | Insulin | >0.05 | Probiotics were ineffective in reducing the incidence of HCAI |
| [50] | Italy | 220 | 10 months | RPCDB | Common diseases | L. rhamnosus GG | 1010 | NR | One capsule/o.d/duration of hospitalization | Inert oligosaccharides | >0.05 | Probiotics were ineffective in preventing nosocomial rotavirus infections |
| [51] | Italy | 90 | 2.9 | RCT | Previously healthy children were admitted with any cause | L. rhamnosus GG | 3 × 109 | NR | L. GG, vitamin B (B1 1.10 mg, B2 and B6 1.40 mg, B12 1.25 lg), vitamin C (40 mg) and zinc (5 mg)/o.d/15 days | Not contain L. GG, vitamins B or C or zinc | <0.05 | Probiotics reduced the incidence of HCAI |
| [52] | Poland | 54 | 11.5 ± 9.2 | RDBPCT | Children, 1–48 months, reasons other than diarrhea | L. reuteri DSM 17938 | 108 | NR | Five drops/o.d/duration of hospitalization | NR | >0.05 | Probiotics did not significantly affect the risk of developing nosocomial diarrhea |
| [53] | Croatia | 727 | 10.23 | RDBPCT | Hospitalized | B. animalis subsp. lactis BB‐12 | 109 | NR | Sachet contained 1 g maltodextrin powder with probiotics/daily in the morning together with breakfast | Maltodextrin | >0.05 | Probiotics failed to prevent nosocomial infections |
| [54] | Brazil | 116 | 62 | RCT | Positive clinical culture and a positive rectal swab for any MDR Gram‐negative bacilli | L. bulgaricus | 1010 | Fructo‐oligosaccharides | Suspended in FOS/b.i.d/7 days |
Similar regime |
>0.05 | Probiotics were ineffective for the decolonization of MDR Gram‐negative bacilli in hospitalized patients |
| L. rhamnosus | 1010 | |||||||||||
| [55] | Sweden | 80 | 68 | RPCCT | ESBL positive | L. plantarum | 4.5 × 1011 | NR | Two Sachets/every morning and every evening/2 months | Maltose and silicon dioxide | 0.24 | Probiotics did not support decolonization but were limited in power |
| L. paracasai | ||||||||||||
| L. acidophilus | ||||||||||||
| L. delbrueckii subsp. Bulgaricus | ||||||||||||
| B. longum | ||||||||||||
| B. infantis | ||||||||||||
| B. breve | ||||||||||||
| S. thermophiles | ||||||||||||
| [56] | USA | 103 | 65 | RCPS | Medical or coronary ICUs | L. rhamnosus GG | 1010 | NR | One capsule/b.i.d/14 days or until study exit | SOC group | >0.05 | Probiotics did not prevent the gastrointestinal colonization of MDR organisms in ICU patients |
| [57] | Sweden | 117 | 76 | RCCT | Obtaining the first OPS within 24 h of hospital admission, and an expected length of stay of more than 72 h | L. plantarum 299v | 1010 | NR | With 3 g of maltodextrin/b.i.d/hospital stay | Maltodextrin | >0.05 | Probiotics were ineffective in regulating the oropharyngeal microbiota |
| L. plantarum 299v | 1010 | |||||||||||
| [58] | Australia | 36 | 56.2 | PRO, RBT | Gastric tube feeding, ICU, diarrhea | L. rhamnosus GG | 1010 | NR | Two capsules/b.i.d/7 days | Inulin | >0.05 | Probiotics were ineffective on the duration or severity of diarrhea |
| [59] | India | 50 | 41 ± 20.72 | RDBPCT | AP | L. acidophilus | 2.5 × 109 | NR | Four Sachets/o.d/7 days | NR | >0.05 | Probiotics were ineffective on gut permeability or endotoxemia in AP patients |
| B. longum | ||||||||||||
| B. bifidum | ||||||||||||
| B. infantis | ||||||||||||
| [60] | Japan | 12 | 84.4 | DBCS |
Bed‐ridden in‐patients over 70 years of age, dysphasia with dementia and fed total EN by nasogastric tube or gastrostomy |
Lactobacillus johnsonii La1 (NCC533) | 109 | NR | Ninety grams (373 kJ [89 kcal]) fermented milk through a tube after feeding of EN at 3395 kJ/o.d/12 weeks | 373 kJ/days of the same EN from the fermented milk | <0.05 | Suppress infections by improving nutritional and immunological status in the elderly |
| [61] | Australia | 218 | 62.1 ± 15.7 | MPPCRCT | Adults within 48 h of ICU admission require ICU care beyond the calendar day after recruitment | L. plantarum 299v | 2 × 1010 | NR | Capsule/o.d/60 days | Microcrystalline cellulose | >0.05 | Probiotic therapy with L. plantarum 299v to adult patients admitted to the ICU is safe but not associated with improved patient outcomes |
| [62] | Japan | 60 | 65.9 ± 8.2 | PRO, RCT | CRC | B. longum | 5 × 1010 | NR | One sachet/o.d/7–14 days preoperatively and 14 days postoperatively | NR | <0.05 | Probiotics contributed to a balanced intestinal microbiota and attenuated postoperative inflammatory responses |
Abbreviations: AAD, antibiotic‐associated diarrhea; AB, antibiotics; AP, acute pancreatitis; b.i.d, twice daily; CAD, Clostridioides difficile associated diarrhea; CDD, Clostridioides difficile diarrhea; CDI, Clostridioides difficile infection; CRC, colorectal cancer; DBCRPCT, double‐blind, concealed randomized, placebo‐controlled trial; DBPCT, double blinded placebo‐controlled trial; DBRCT, double‐blind randomized controlled trial; DBRPCCT, double‐blind randomized placebo controlled clinical trial; ESBL, extended spectrum beta‐lactamases; FOS, fructo‐oligosaccharide; GCSs, Glasgow Coma Scale score; ges., gestational age; HCAI, nosocomial infections; IV, intravenous; MDBPS, multicenter double‐blind prospective study; M.O, microorganisms; MU, multicenter; NEC, necrotizing enterocolitis; NICU, newborn intensive care unit; NR, not reported; o.d, once daily; OLRCT, open‐label randomized controlled trial; OPS, oropharyngeal swabs; PICU, pediatric intensive care unit; PRO, prospective; RBT, randomized blinded trial; RCCT, randomized controlled clinical trial; RCMT, randomized controlled multicenter trial; RCOT, randomized controlled open trial; RCPS, randomized controlled pilot study; RDBPCT, randomized double‐blind placebo‐controlled trial; RDBPMT, randomized double‐blind placebo‐controlled multicenter trial; RPCCT, randomized placebo‐controlled clinical trial; RPCDB, randomized, placebo‐controlled double‐blind; RPCT, randomized placebo‐controlled trial; RPS, randomized pilot study; SOC, standard of care; s.t.d, six time a day; TBI, traumatic brain injury; t.i.d, three time a day; UTI, urinary tract infections; VAP, ventilator‐associated pneumonia; VLBW, very low birth weight.
A total of 24 probiotic species were administered once, twice, or thrice daily at 1 × 108 to 4.5 × 1011 colony forming units (CFU) and an optimum dose of 4.65 × 1010 CFU. Lactobacillus rhamnosus (14.51%; n = 18) was the most common probiotic used by different studies (Figure 2). As shown in Table 1, among the 54 clinical trials, 23 trials used multistrain probiotic regimens, so that five trials used two types of probiotic bacteria, six trials used two types, six trials used four types, two trials used seven types, two studies used eight types, and one study used three types of probiotic bacteria in combination. On the other hand, 31 trials used single‐strain probiotic regimens, 4 trials used synbiotics and one trial used prebiotics.
Figure 2.

The frequency of probiotic species used in various clinical trials for patients with infectious diseases.
3.1. Effects of probiotics on the prevention of AAD and CDI
The type and formula of the probiotics were ignored when determining the total effects of probiotics. Twenty clinical trials 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 assessed the effects of probiotics on the reduction or prevention of AAD/CDI. In total, 4262 participants were in the probiotic group and 4015 in the placebo group. Among these 20 trials, 5 trials were on adults ≥18 years, 3 trials were performed on adults aged 50 years and older, two trials were on children ≤14 years, two trials were on children ≤12 years, one trial was on adults ≥42 years, one trial was on adults ≥65 years, one trial on younger adults <18 years, one trial was on adults between 25 and 50 years, two trial was on adults 30 to 70 years, and two trials did not mention the participant's age. In addition, nine trials used single‐strain probiotic regimens, five used three types of probiotic bacteria, five used two species, and one used VSL#3 containing eight probiotic species. Seven out of 20 clinical trials indicated the significant role of probiotics in reducing AAD (p < 0.05). Moreover, the mean prevalence of AAD in receiving probiotics (10.6%) was significantly lower than the placebo group (34.5%) (p < 0.05), as well as, the lower prevalence of CDI in the probiotic group (2.3%) than the placebo group (15.6%) (p < 0.05). Hickson et al. 11 also highlighted the considerable effect of probiotics on the reduction of AAD in receiving probiotics than placebo in patients over 50 years (12% vs. 34%, p < 0.05). In addition, they found CDI only in the placebo group (17%). Nevertheless, 13 clinical trials failed to show any beneficial effects of the probiotics in the reduction of AAD and CDI in the probiotic and placebo groups (p > 0.05).
The percentage of antibiotic consumption in the placebo and probiotic groups, based on the antibiotic class, is shown in Figure 3. Moreover, beta‐lactams, macrolides, quinolones, aminoglycosides, and tetracyclines were used in 15, 11, 7, 5 and 3 trials, respectively. According to these results, the exposure of the placebo and probiotic groups to the different classes of antibiotics was the same. The mean duration of hospitalization was shorter in the probiotic group compared to the placebo group (8.4 days vs. 9.6 days, p > 0.05). However, the mean duration of antibiotic treatment was relatively the same in both the probiotic and placebo groups (8.76 days vs. 9.04 days, p > 0.05, respectively). Some complications were seen in both probiotic and placebo groups, with the mean prevalence of 55.3% and 56.6%; p > 0.05, respectively. Moreover, Allen et al. 10 and Beausoleil et al. 16 indicated the frequency of some common compliances so that the mean prevalence of nausea, bloating, vomiting, flatus, abdominal pain, and tenesmus was 7.8%, 8.7%, 4.25%, 8.5%, 11.4%, and 3% in the probiotic group, and 7.1%, 8.25%, 9.1%, 6.2%, 12.15%, and 1.85% in the placebo group, respectively. Also, Barker et al. 9 evaluated the effects of probiotics on 33 patients with mild to moderate C. difficile infection and indicated that the total number of days with diarrhea was considerably shorter in the probiotic group than in the placebo group (3.5 vs. 12.0 days; p < 0.05). However, there was no significant difference (p > 0.05) in the rate of CDI recurrence or functional improvement over time between the two groups.
Figure 3.
Percentage of antibiotic consumption in the placebo and probiotic groups regarding the antibiotic class.
3.2. Effects of probiotics in the prevention of VAP
The type and formula of the probiotics were ignored when determining the total effects of probiotics. According to the obtained results, 10 clinical trials 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 evaluated the effects of probiotics, and two trials evaluated the effects of synbiotics on VAP hospitalized in ICU. Among these trials, 11 had investigated these effects on the adult population 29 , 30 , 31 , 32 , 34 , 35 , 36 , 37 , 38 , 39 , 40 and one on children. 33 In total, 2132 individuals were in the probiotic group and 2032 in the placebo group.
The indications of surgical, trauma, and medical ICU patients were 453, 526, and 2730, respectively. The feeding modalities/nutritional status was usually enteral feeding by nasogastric tube (9 RCTs), nasal tube (1 RCT), duodenal/gastric tube (1 RCT), and oropharyngeal tube (1 RCT).
In total, six clinical trials 29 , 31 , 32 , 33 , 35 , 36 highlighted the considerable effects of probiotics on the reduction or prevention of VAP (p < 0.05), so that the mean prevalence of VAP was lower in the probiotic group (23.89%, ranging from 0.66% to 40.7%) than the placebo group (38.27%, ranged 0.94% to 53%). However, some studies 30 , 34 , 37 , 38 , 39 , 40 did not find any effects following probiotic consumption.
Banuperiya et al. 33 in children and Mahmoodpoor et al. 29 and Tan et al. 39 in adults indicated that the mean length of ICU stay days of VAP patients was significantly shorter in the probiotic group than the placebo group (9.03 vs. 13.93 days; p < 0.05). In addition, Banuperiya et al. 33 and Mahmoodpoor et al. 29 found a significant difference in the mean length of hospital stay days between probiotic and placebo groups (13.75 vs. 20.4; p < 0.05 days). However, other studies 30 , 31 , 32 , 34 , 35 , 37 , 38 , 40 did not find any beneficial effect (p > 0.05) after the consumption of probiotics compared to the placebo group on the reduction of length of ICU or hospital stay. Knight et al. 38 and Zeng et al. 32 indicated that the mortality rate of ICU and hospital of VAP patients in the probiotic group had no significant difference as compared to that in the placebo group (p < 0.05). Tan et al. 39 Barruad et al. 37 and Rongrungruang et al. 30 showed that the mortality on Day 28 and Day 90 had no difference (p > 0.05) between the probiotic and placebo groups. Also, other studies did not find an effect on the reduction of the total mortality percentage between probiotic and placebo groups.
The frequency rates of bacteria causing VAP, characterized by the microbiological culture of bronchoalveolar lavage, oropharynx, blood, or tracheal aspirate samples, were considerably higher in the probiotic group compared to that in the placebo group (p < 0.05).
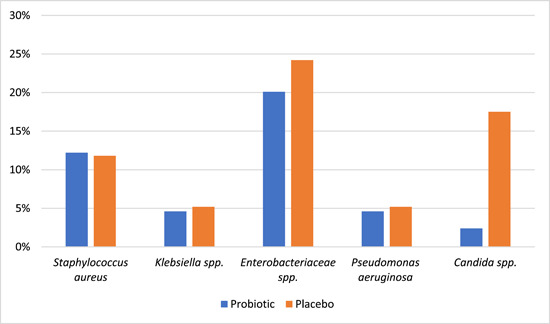
Zeng et al. 32 indicated that the rates of gastric colonization of the potentially pathogenic microorganisms including Enterobacteriaceae, non‐fermentative Gram‐negative bacteria, Enterococcus spp., Staphylococcus aureus, Streptococcus spp. and Candida spp. were considerably lower in the probiotics group (24%) compared to the placebo group (44%) (p = 0.004). However, probiotics did not improve the eradication of gastric colonization with these microorganisms compared to the placebo group (27.8% vs. 19.2%; p = 0.756). Shimuzo et al. 31 indicated, by the analysis of faecal microbiota among the VAP patients, that the number of Bifidobacterium spp., Lactobacillus spp., and Atopobium clusters significantly increased during the first and second weeks of synbiotic consumption compared to those in the no‐synbiotics group (p < 0.05). Also, Mahmoodpoor et al. 29 indicated that consumption of probiotics caused a nonsignificant decrease (p > 0.05) in the diarrhea prevalence, gastric colonization, and incidence of multidrug‐resistant pathogens among the VAP patients compared to those in the placebo group. Morrow et al. 35 indicated that the probiotic usage in the patients with confirmed VAP led to a significant reduction in the rate of C. difficile diarrhea in the probiotic group compared to the control group (18.6% vs. 5.8%, respectively; p = 0.02). In addition, the duration of C. difficile diarrhea was considerably lower among the patients receiving Lactobacillus therapy compared to the control group (4.1 days vs. 5.9 days, respectively; p = 0.03).
3.3. Effects of probiotics on the prevention of nosocomial infections among the preterm infants
According to the results, three clinical trials 41 , 42 , 43 assessed the effects of probiotics on the prevention of nosocomial infections, including urinary tract infection (UTI), pneumonia, meningitis, and sepsis among the preterm infants. In total, 813 participants were in the probiotic group and 821 in the placebo group. The mean total rate of nosocomial infections was nonsignificantly higher in the probiotic group compared to the control group (27.5% vs. 24.3%, respectively; p > 0.05). Dani et al. 41 indicated that the prevalence rate of UTI was nonsignificantly lower in the probiotic group compared to the control group (3.4% vs. 5.3%; p > 0.05), whilst the rate of sepsis was higher in the probiotic group (4.7%) rather than the control group (4.1%). On the other hand, Rojas et al. 43 demonstrated a higher prevalence of pneumonia in the probiotic group compared to the control group (5% vs. 2.4%; p > 0.05); also the rate of meningitis was similar in both groups (0.3%). Westerbeek et al. 44 indicated that the enteral supplementation of a prebiotic mixture consisting of neutral oligosaccharides caused a lower incidence of ≥1 severe endogenous infection and ≥2 serious infectious episodes in the prebiotic group than in the placebo group (p = 0.09 and p = 0.07, respectively).
3.4. Effects of probiotics on the prevention of common nosocomial infections among hospitalized infants and children
The type and formula of the probiotics were ignored when determining the total effects of probiotics. A total of nine clinical trials 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 assessed the effects of probiotics on the reduction or prevention of nosocomial infections among hospitalized children. Moreover, seven clinical trials were performed inwards of non‐ICU, and two clinical trials were performed on children and infants hospitalized in ICU. Also, only two out of nine clinical trials used breastfeeding in some infants. In total, 839 participants were in the probiotic group and 824 in the placebo group. The age range of children was from birth to 6 years. Four clinical trials 45 , 47 , 48 , 51 indicated the considerable effects of probiotics on the reduction or prevention of nosocomial infections among hospitalized infants and children compared to placebo (10.9% vs. 29.67% days; p < 0.05). They also highlighted that the duration of hospitalization days was significantly shorter in the probiotic than in the placebo group (9.35 vs. 12.28 days; p < 0.05).
Moreover, Szajewska et al. 47 indicated that the prophylactic use of Lactobacillus GG significantly reduced the risk of nosocomial diarrhea in infants (6.7% vs. 33.3%, p < 0.05), particularly nosocomial rotavirus gastroenteritis (2.2% vs. 16.7%, p < 0.05) in the probiotic group than the placebo group, respectively.
However, other clinical trials 46 , 49 , 50 , 52 , 53 did not find the beneficial effects on the consumption of probiotics against nosocomial infections or decreasing the length of hospitalization days in children.
Wanke et al. 52 and Mastretta et al. 50 indicated that two probiotic strains of Lactobacillus reuteri DSM and Lactobacillus GG did not have any beneficial effect on rotavirus infections. Moreover, Mastretta et al. 50 indicated that the attack rate of rotavirus infections among the infants who received probiotics was lower than the placebo group (25.4% vs. 30.2%); however, this difference was not significant (p > 0.05). In addition, the attack rate of rotavirus infections among breastfed infants was lower than non‐breastfed infants (10.6% vs. 32.4%) and this difference were significant (p < 0.05). However, the probiotic consumption did not have any beneficial effect on the attack rate of rotavirus infections in either breastfed or non‐breastfed infants (p > 0.05).
3.5. Effects of probiotics on the prevention of infections associated with multidrug resistance (MDR) and extensive spectrum beta‐lactamase (ESBL)‐producing bacteria
Three clinical trials 54 , 55 , 56 evaluated the effects of probiotics on the prevention of MDR, ESBL, and VRE infections. Ljungquist et al. 55 evaluated the effect of Vivomixx® (daily consumption in 2 months), as a probiotic regimen, on the eradication of intestinal extended‐spectrum b‐lactamase (ESBL)‐producing Enterobacteriaceae among the patients harboring these organisms. Rectal swabs cultured at the end of a 1‐year follow‐up were used to determine the effects of this probiotic mixture. According to the results, 12.5% of patients in the probiotic group and 5% of patients in the placebo group had successfully decreased the intestinal rate of ESBL‐producing Enterobacteriaceae; however, this decrease was not statistically significant (p = 0.24). Salomao et al. 54 investigated the effectiveness of a synbiotic mixture (L. bulgaricus, L. rhamnosus, and fructo‐oligosaccharides), as the eradication therapy for patients with prolonged intestinal multidrug‐resistant (MDR) Gram‐negative infection, by the culture of rectal swabs. According to the results, MDR gram‐negative bacilli were higher in the placebo compared to the symbiotic group (20.7% vs. 16.7%, respectively; p = 0.60). Also, Kwon et al. 56 indicated no significant difference in the overall acquisition of any MDR organism between the probiotic and placebo groups (10% vs. 15%, respectively; p = 0.72).
These three studies showed that the consumption of symbiotic or probiotic mixtures was ineffective for the intestinal eradication of MDR or ESBL‐producing gram‐negative bacilli.
3.6. Effects of probiotics on the prevention of nosocomial viral infections associated with diarrhea
Five clinical trials 12 , 46 , 47 , 50 , 52 investigated the effects of probiotics on the eradication of rotavirus nosocomial infections and one clinical trial on norovirus. 10 Moreover, these five clinical trials indicated that the application of probiotics did not have any significant effects (p > 0.05) on the prevention of rotavirus nosocomial infections. On the other hand, Allen et al. 10 evaluated the efficacy of probiotic consumption on the rate of norovirus‐associated diarrhea and indicated a similar rate among the probiotic and placebo groups (0.4%).
In recent years, several RCTs have assessed the effects of probiotics on the clinical consequences of infectious diseases among ICU and non‐ICU patients. Accordingly, the present qualitative systematic review was designed to evaluate and summarize the findings of these RCTs.
In RCTs included in this systematic review, probiotics (i.e., bacteria and fungi) from different genera have been studied, including Lactobacillus spp., Bifidobacterium spp., Streptococcus spp., Enterococcus spp., Bacillus spp., Pediococcus spp., and Saccharomyces spp., among which, L. rhamnosus was the most widely applied probiotic (18 out of 54 trials, 33.33%). In 6 out of the 18 studies, prescription of this probiotic bacteria was correlated with the prevention or reduction of the VAP incidence, 29 , 33 , 35 a decrease in the length of ICU and hospital stay, 29 a decline in the incidence of healthcare‐associated infection (HCAI), 51 and reduce the risk of nosocomial diarrhea in infants, particularly nosocomial rotavirus gastroenteritis. 47 However, applying its supplements did not impact the clinical outcomes of patients in other RCTs. Besides, statistical analysis of the results showed that the optimal dose of this bacterium was 4.65 × 1010 CFU. Among the included RCTs, there was a high diversity in the number of species (single or multiple species) and the quantity of prescribed daily doses (1 × 108 to 4.5 × 1011 CFUs). They also had differences in the administration routes (i.e., capsule, sachet, fermented dairy, lyophilized powder, and drop). These variations can play a substantial role in causing differences in the results of various studies and render it challenging to interpret the outcomes. Some previous systematic review and meta‐analysis studies 63 , 64 reported the results of RCTs, in which the L. rhamnosus supplementation was beneficial in reducing some infectious diseases in children such as acute otitis media, upper respiratory tract infections, health‐care‐associated diarrhea, and symptomatic rotavirus gastroenteritis.
Regarding the high burden of CDI in hospitals, finding a way to lower the rate or duration of CDI and AAD is relevant and can prevent the transmission of C. difficile as well as inappropriate antibiotic prescription and therapeutic costs. 9 , 11 Therefore, studies evaluating the efficacy of probiotics on AAD and CDI are of great importance. In the current systematic review, 20 clinical trials studied the impact of probiotics on the prevention or reduction of AAD and CDI and reported controversial results. Seven out of the 20 clinical trials (35%) confirmed the significance of probiotics in decreasing the incidence rate of AAD or CDI, but in 12 studies (65%), no positive effects were observed. The investigated patients in both probiotic and placebo groups had the same exposure to the antibiotic classes, and the mean duration of antibiotic therapy was similar for both groups. Therefore, these factors did not affect the results. Some previous systematic reviews published consistent results and demonstrated a noticeable decrease in the risk of AAD and CDI due to probiotic administration. 65 , 66 In a recently published meta‐analysis study by Liao et al. 67 probiotic consumption resulted in a 38% reduction in AAD incidence rate in adult patients. The authors concluded that consuming probiotic supplementation at the early stages of antibiotic therapy would be beneficial in preventing AAD occurrence. In another review study, 68 Goldenberg and colleagues evaluated the preventive effect of probiotics on CDI in adults and children. Their results revealed that probiotics are effective for CDI prevention, and their short‐term use seems safe and efficacious in combination with antibiotics. Probiotics can inhibit the occurrence of AAD or CDI in some ways, including their potency in replacing the modified intestinal microflora, which results in the inhibition of intestine colonization by pathogens and the production of antitoxic or antimicrobial compounds. 13 , 69 There are some reasons for the discrepancies observed in the results of RCTs. First, these studies were different regarding the type of antibiotics used by the patients, duration of treatment, type of probiotic bacteria, daily doses, and the age of participants. Second, the sensitivity of probiotic strains to the antibiotic regimens consumed by the patients can influence the effectiveness of probiotics. For example, in the study conducted by Mantegazza et al. 65 the sensitivity of L. rhamnosus GG to penicillin was noticed as a factor that influences L. rhamnosus GG efficacy in the prevention of AAD. Third, the susceptibility of the studied probiotic bacteria to gastric acid and bile salts is a barrier to the survival of these bacteria in the gastrointestinal tract, which ultimately affects their effectiveness in preventing or reducing AAD and CDI. 14
VAP is another infection in which the role of probiotics is investigated. In this study, six RCTs (including two RCTs on synbiotics and four RCTs on probiotics) indicated the significant role of probiotics in reducing the rate of VAP, the mean length of ICU stays, the mortality rate, and the duration of mechanical ventilation. In addition, the use of probiotics and synbiotics had other effects, including changes in the composition of fecal microbiota that alters the rate of gut colonization with the pathogenic bacteria, reducing the incidence and the duration of CDI, as well as the production of acetate that decreases inflammation, and septic complications. Although these RCTs reported the positive effects of probiotic consumption, they did not see any significant differences in the mortality rate of VAP patients among the probiotic and placebo groups. In a recent systematic review and meta‐analysis study conducted by Zhao et al. 69 probiotic treatments contributed to the considerable reduction of VAP and did not change the mortality rate. However, in contrast to our study, they did not report any statistically significant differences in the length of mechanical ventilation, duration of ICU hospitalization, and mortality rate. Batra et al. 70 performed a systematic review and meta‐analysis and reported similar results to our study. On the contrary, some meta‐analysis studies found no positive association between probiotics and reduction of VAP incidence. 71 , 72 These discrepancies may be due to the small sample size, the short length of the study, the weak immune system of ICU patients, and differences in the feeding routes of patients by the probiotics (i.e., nasogastric tube, nasal tube, duodenal/gastric tube, and oropharyngeal tube), the presence of underlying diseases, 32 and differences in the diagnostic criteria for establishing VAP (microbiological or clinical methods). 35
Among the nine RCTs that investigated the impact of probiotics on nosocomial infections in children and infants, only four studies (44.4%) detected a significant correlation between probiotic consumption and the reduction of these infections. 45 , 47 , 48 , 51 In addition, probiotic therapy led to the shortening of the length of hospital stay. However, it did not affect the attack rate of rotavirus gastroenteritis. These studies were different in some aspects, including the sample size, type of probiotics, probiotic doses, type of the studied infections, wards where patients were admitted (ICU or non‐ICU), and type of infant feeding (breastfeeding or formula), which can cause conflicting findings. In a review study published in 2017, 73 Hojsak discussed the effect of probiotics on children and suggested that L. rhamnosus GG is efficacious for preventing hospital‐acquired diarrhea and respiratory tract infections in daycare centers. It is important to note that the influences of probiotics are species‐specific, and not all types of probiotics are suitable for fighting different infections. 53 Consequently, the proper choice of probiotics is critical. In addition, the optimum dose for most probiotics is undetermined, so using lower doses will result in incorrect conclusions. 52
Three RCTs assessed the impacts of probiotics on the prevention of nosocomial infections in preterm infants and described contradictory outcomes. According to the results, the incidence rate of UTI, sepsis, and meningitis was relatively similar in both the probiotic and placebo groups. However, significant differences were observed in the incidence rate of pneumonia in probiotic (5%) and placebo (2.4%) groups. Olsen et al. 74 evaluated 12 RCTs and showed that probiotics decreased the chance of necrotizing enterocolitis (NEC) and death in preterm infants. In a review study by AlFaleh et al. 75 the safety and efficacy of probiotics in preterm infants were investigated. They evaluated 19 RCTs and concluded that enteral probiotic supplementation remarkably lowered the incidence of severe NEC and mortality in premature infants. However, they did not see any significant decrease in nosocomial sepsis, which is consistent with our findings. Lack of probiotics effects on nosocomial infections in infants can occur for various reasons, including the improper dose of probiotics and the adverse influence of antibiotics, which causes insufficient growth of probiotics in the intestine of infants. 43
Three studies investigated the efficacy of synbiotic or probiotic mixtures on the intestinal eradication of MDR or ESBL‐producing gram‐negative bacilli, and the results did not show any positive effects. The effect of probiotics could be due to the colonization resistance that inhibits the colonization of enteric epithelium by other pathogens. Besides, they can digest metabolic precursors and produce short‐chain fatty acids (SCFAs) that result in immune modulation and increase the barrier effect of the mucosa. Moreover, these bacteria produce antimicrobial compounds. 76 Poor outcomes obtained in these studies can be due to several reasons, such as short study time, incorrect choice of the studied strains, and the use of antibiotics that harmed the probiotic strains.
Prevention of viral‐nosocomial infections by probiotics is another area of interest for researchers, and in the current systematic review, six RCTs have investigated this topic. All the clinical trials showed that probiotic treatment was ineffective in preventing rotavirus/norovirus nosocomial infections. Several studies reviewed the influence of probiotics on rotavirus nosocomial infection. Szajewska et al. 77 demonstrated that utilizing L. rhamnosus GG decreased the rate of symptomatic rotavirus gastroenteritis in children. However, Mastretta et al. 50 indicated a nonsignificant effect of probiotic treatment on nosocomial rotavirus diarrhea. Probiotics increase humoral responses against rotavirus infection, which may contribute to the prevention of rotavirus nosocomial infection by probiotics. 78
Considering the vulnerability and difficulty of treating elderly patients (i.e., patients >65 years or older), we have evaluated the results of RCTs on this age group. Out of 54 RCTs that were included in this study, 20 studies assessed the efficacy of different probiotic species on elderly patients. Only six of these studies (30%) reported positive effects of probiotic consumption, including their ability to prevent or treat AAD or CDI, 9 , 11 , 16 modulate gut microbiota and prevent VAP and enteritis, 31 suppress infection by improving the nutritional and immunological status of patients, 67 and attenuate postoperative inflammatory responses. 62 However, these results were not statistically significant (p > 0.05). Moreover, the remaining 14 studies did not find promising results. 10 , 13 , 23 , 24 , 30 , 34 , 37 , 40 , 54 , 55 , 56 , 57 , 61 In a systematic review and meta‐analysis study performed by researchers from Samuel Merritt University, 79 the effectiveness of probiotics in reducing the incidence of CDI in elderly hospitalized patients was examined. The study included randomized controlled trials involving patients aged 60 years and older who were residing in acute and post‐acute care facilities and undergoing or about to undergo antibiotic treatment for managing various infectious diseases, except for CDI. They evaluated five RCTs, and analysis of their results did not support the efficacy of probiotics in decreasing the incidence of CDI in elderly patients. A systematic review and meta‐analysis study conducted by Jafarnejad et al. 80 from Iran investigated the role of probiotics in reducing the risk of AAD in two age groups; adults (18–64 years) and elderly (>65 years) patients. In total, 30 RCTs were included in this study, and a meta‐analysis of their results demonstrated that probiotics did not affect AAD incidence in elderly patients. It is worth noting that the negative results obtained in previous RCTs may be attributed to the lack of attention to the specific characteristics of elderly patients and the selection of inappropriate probiotic treatment regimens for this population. According to the literature, the gut microbiota of elderly patients has lower bacterial diversity, with a lower number of beneficial microorganisms (such as Firmicutes, especially Clostridium cluster XIVa and Faecalibacterium prausnitzii) and an increased number of facultative anaerobic bacteria, such as Proteobacteria. Numerous investigations have reported a link between increased age and a decline in the number of Bacteroides. Elderly individuals may also have reduced dentition and chewing strength and a loss of appetite, which can lead to a limited variety of food ingredients that support microbial diversity. These changes result in decreased production of SCFAs and a shift from a predominantly saccharolytic metabolism (typically found in adults) to a predominantly putrefactive metabolism. 81 Therefore, selecting the appropriate probiotic regimen for this age group can result in treatment success.
4. CONCLUSIONS
This systematic review presents information on the average advantage of probiotics in preventing or reducing VAP in ICU patients and children with CDI, AAD, and nosocomial infections. These beneficial effects seem to be achieved if the relevant probiotic species and doses are selected in adjunction with antibiotics. This systematic review does not support the positive impacts of these bacteria on the prevention of nosocomial infections in preterm infants, the intestinal eradication of MDR, ESBL gram‐negative bacilli, or rotavirus nosocomial infection. Further studies are required to estimate the role of probiotics in combating these infections and assess the variables. None of the reviewed RCTs reported any adverse side effects, reflecting the reasonable safety of these organisms.
AUTHOR CONTRIBUTIONS
Atieh Darbandi: Conceptualization; data curation; writing—original draft. Maryam Banar: Conceptualization; data curation; software. Maryam Koupaei: Conceptualization; data curation; formal analysis; investigation. Roghayeh Afifirad: Investigation; software; validation; writing—original draft. Parisa Asadollahi: Software; supervision; validation; writing—original draft. Elnaz Bafandeh: Investigation; software. Iraj Rasooli: Resources; supervision; validation. Amir Emamie: Investigation; methodology; visualization. Tahereh Navidifar: Resources; software; supervision; validation; visualization. Parviz Owlia: Supervision; validation; visualization; writing—original draft; writing—review & editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Tahereh Navidifar, Parviz Owlia affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Darbandi A, Banar M, Koupaei M, et al. Clinical efficacy of probiotics in prevention of infectious diseases among hospitalized patients in ICU and non‐ICU wards in clinical randomized trials: a systematic review. Health Sci Rep. 2023;6:e1469. 10.1002/hsr2.1469
Contributor Information
Tahereh Navidifar, Email: roya_67@ymail.com.
Parviz Owlia, Email: owliaparviz@gmail.com, Email: powlia@shahed.ac.ir.
DATA AVAILABILITY STATEMENT
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Hill C, Guarner F, Reid G, et al. The International Scientific Association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506‐514. [DOI] [PubMed] [Google Scholar]
- 2. Chapman CMC, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr. 2011;50(1):1‐17. [DOI] [PubMed] [Google Scholar]
- 3. Vieco‐Saiz N, Belguesmia Y, Raspoet R, et al. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food‐animal production. Front Microbiol. 2019;10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bermudez‐Brito M, Plaza‐Díaz J, Muñoz‐Quezada S, Gómez‐Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61(2):160‐174. [DOI] [PubMed] [Google Scholar]
- 5. Trafalska E, Grzybowska K. Probiotics—an alternative for antibiotics?. Wiad Lek. 2004;57(9‐10):491‐498. [PubMed] [Google Scholar]
- 6. Kotzampassi K, Giamarellos‐Bourboulis EJ. Probiotics for infectious diseases: more drugs, less dietary supplementation. Int J Antimicro Ag. 2012;40(4):288‐296. [DOI] [PubMed] [Google Scholar]
- 7. Liu Y, Tran DQ, Rhoads JM. Probiotics in disease prevention and treatment. J Clin Pharmacol. 2018;58(suppl 10):S164‐S179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barker AK, Duster M, Valentine S, et al. A randomized controlled trial of probiotics for Clostridium difficile infection in adults (PICO). J Antimicrob Chemother. 2017;72(11):3177‐3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic‐associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2013;382(9900):1249‐1257. [DOI] [PubMed] [Google Scholar]
- 11. Hickson M, D'Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335(7610):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kołodziej M, Szajewska H. Lactobacillus reuteri DSM 17938 in the prevention of antibiotic‐associated diarrhoea in children: qa randomized clinical trial. Clin Microbiol Infect. 2019;25(6):699‐704. [DOI] [PubMed] [Google Scholar]
- 13. Pozzoni P, Riva A, Bellatorre AG, et al. Saccharomyces boulardii for the prevention of antibiotic‐associated diarrhea in adult hospitalized patients: a single‐center, randomized, double‐blind, placebo‐controlled trial. Am J Gastroenterol. 2012;107(6):922‐931. [DOI] [PubMed] [Google Scholar]
- 14. Thomas MR, Litin SC, Osmon DR, Corr AP, Weaver AL, Lohse CM. Lack of effect of Lactobacillus GG on antibiotic‐associated diarrhea: a randomized, placebo‐controlled trial. Mayo Clin Proc. 2001;76(9):883‐889. [DOI] [PubMed] [Google Scholar]
- 15. Selinger CP, Bell A, Cairns A, Lockett M, Sebastian S, Haslam N. Probiotic VSL#3 prevents antibiotic‐associated diarrhoea in a double‐blind, randomized, placebo‐controlled clinical trial. J Hosp Infect. 2013;84(2):159‐165. [DOI] [PubMed] [Google Scholar]
- 16. Beausoleil M, Fortier N, Guénette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic‐associated diarrhea: a randomized, double‐blind, placebo‐controlled trial. Can J Gastroenterol. 2007;21(11):732‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Can M, Beşirbellioglu BA, Avci IY, Beker CM, Pahsa A. Prophylactic Saccharomyces boulardii in the prevention of antibiotic‐associated diarrhea: a prospective study. Med Sci Monit. 2006;12(4):19‐22. [PubMed] [Google Scholar]
- 18. Ehrhardt S, Guo N, Hinz R, et al. Saccharomyces boulardii to prevent Antibiotic‐associated diarrhea: a randomized, double‐masked, placebo‐controlled trial. Open Forum Infect Dis. 2016;3(1):ofw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose‐response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic‐associated diarrhea and Clostridium difficile‐associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105(7):1636‐1641. [DOI] [PubMed] [Google Scholar]
- 20. Georgieva M, Pancheva R, Rasheva N, Usheva N, Ivanova L, Koleva K. Use of the probiotic Lactobacillus reuteri dsm 17938 in the prevention of antibiotic‐associated infections in hospitalized Bulgarian children: a randomized, controlled trial. J of IMAB. 2015;21(4):895‐900. [Google Scholar]
- 21. Lönnermark E, Friman V, Lappas G, Sandberg T, Berggren A, Adlerberth I. Intake of Lactobacillus plantarum reduces certain gastrointestinal symptoms during treatment with antibiotics. J Clin Gastroenterol. 2010;44(2):106‐112. [DOI] [PubMed] [Google Scholar]
- 22. Fox MJ, Ahuja KDK, Robertson IK, Ball MJ, Eri RD. Can probiotic yogurt prevent diarrhoea in children on antibiotics? A double‐blind, randomised, placebo‐controlled study. BMJ Open. 2015;5(1):e006474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song HJ, Kim JY, Jung SA, et al. Effect of probiotic Lactobacillus (Lacidofil® cap) for the prevention of antibiotic‐associated diarrhea: a prospective, randomized, double‐blind, multicenter study. J Korean Med Sci. 2010;25(12):1784‐1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rajkumar C, Wilks M, Islam J, et al. Do probiotics prevent antibiotic‐associated diarrhoea? Results of a multicentre randomized placebo‐controlled trial. J Hosp Infect. 2020;105(2):280‐288. [DOI] [PubMed] [Google Scholar]
- 25. Ruszczyński M, Radzikowski A, Szajewska H. Clinical trial: effectiveness of Lactobacillus rhamnosus (strains E/N, Oxy and Pen) in the prevention of antibiotic‐associated diarrhoea in children: clinical trial: L. rhamnosus in the prevention of antibiotic‐associated diarrhoea. Aliment Pharmacol Ther. 2008;28(1):154‐161. [DOI] [PubMed] [Google Scholar]
- 26. Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic‐associated diarrhea—a placebo controlled double‐blind randomized, multi‐center study. Arch Med Sci. 2010;6(1):56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shan LS, Hou P, Wang ZJ, et al. Prevention and treatment of diarrhoea with Saccharomyces boulardii in children with acute lower respiratory tract infections. Benef Microbes. 2013;4(4):329‐334. [DOI] [PubMed] [Google Scholar]
- 28. Ouwehand AC, DongLian C, Weijian X, et al. Probiotics reduce symptoms of antibiotic use in a hospital setting: a randomized dose response study. Vaccine. 2014;32(4):458‐463. [DOI] [PubMed] [Google Scholar]
- 29. Mahmoodpoor A, Hamishehkar H, Asghari R, Abri R, Shadvar K, Sanaie S. Effect of a probiotic preparation on ventilator‐associated pneumonia in critically ill patients admitted to the intensive care unit: a prospective double‐blind randomized controlled trial. Nutr Clin Pract. 2019;34(1):156‐162. [DOI] [PubMed] [Google Scholar]
- 30. Rongrungruang Y, Krajangwittaya D, Pholtawornkulchai K, Tiengrim S, Thamlikitkul V. Randomized controlled study of probiotics containing Lactobacillus casei (Shirota strain) for prevention of ventilator‐associated pneumonia. J Med Assoc Thai. 2015;98(3):253‐259. [PubMed] [Google Scholar]
- 31. Shimizu K, Yamada T, Ogura H, et al. Synbiotics modulate gut microbiota and reduce enteritis and ventilator‐associated pneumonia in patients with sepsis: a randomized controlled trial. Crit Care. 2018;22(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zeng J, Wang CT, Zhang FS, et al. Effect of probiotics on the incidence of ventilator‐associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Intensive Care Med. 2016;42(6):1018‐1028. [DOI] [PubMed] [Google Scholar]
- 33. Banupriya B, Biswal N, Srinivasaraghavan R, Narayanan P, Mandal J. Probiotic prophylaxis to prevent ventilator associated pneumonia (VAP) in children on mechanical ventilation: an open‐label randomized controlled trial. Intensive Care Med. 2015;41(4):677‐685. [DOI] [PubMed] [Google Scholar]
- 34. Klarin B, Molin G, Jeppsson B, Larsson A. Use of the probiotic Lactobacillus plantarum 299 to reduce pathogenic bacteria in the oropharynx of intubated patients: a randomised controlled open pilot study. Crit Care. 2008;12(6):R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator‐associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010;182(8):1058‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giamarellos‐Bourboulis EJ, Bengmark S, Kanellakopoulou K, Kotzampassi K. Pro‐ and synbiotics to control inflammation and infection in patients with multiple injuries. J Trauma: Injury, Infection Crit Care. 2009;67(4):815‐821. [DOI] [PubMed] [Google Scholar]
- 37. Barraud D, Blard C, Hein F, et al. Probiotics in the critically ill patient: a double blind, randomized, placebo‐controlled trial. Intensive Care Med. 2010;36(9):1540‐1547. [DOI] [PubMed] [Google Scholar]
- 38. Knight DJW, Gardiner D, Banks A, et al. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: a randomised, double‐blind, placebo‐controlled trial. Intensive Care Med. 2009;35(5):854‐861. [DOI] [PubMed] [Google Scholar]
- 39. Tan M, Zhu JC, Du J, Zhang LM, Yin HH. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain‐injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnstone J, Meade M, Lauzier F, et al. Effect of probiotics on incident ventilator‐associated pneumonia in critically ill patients: a randomized clinical trial. JAMA. 2021;326(11):1024‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. Neonatology. 2002;82(2):103‐108. [DOI] [PubMed] [Google Scholar]
- 42. Mihatsch WA, Vossbeck S, Eikmanns B, Hoegel J, Pohlandt F. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very‐low‐birth‐weight infants: a randomized controlled trial. Neonatology. 2010;98(2):156‐163. [DOI] [PubMed] [Google Scholar]
- 43. Rojas MA, Lozano JM, Rojas MX, et al. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics. 2012;130(5):e1113‐e1120. [DOI] [PubMed] [Google Scholar]
- 44. Westerbeek EA, van den Berg JP, Lafeber HN, et al. Neutral and acidic oligosaccharides in preterm infants: a randomized, double‐blind, placebo‐controlled trial. Am J Clin Nutr. 2010;91(3):679‐686. [DOI] [PubMed] [Google Scholar]
- 45. Wang Y, Gao L, Zhang YH, Shi CS, Ren CM. Efficacy of probiotic therapy in full‐term infants with critical illness. Asia Pac J Clin Nutr. 2014;23(4):575‐580. [DOI] [PubMed] [Google Scholar]
- 46. Urbańska M, Gieruszczak–Białek D, Szymański H, Szajewska H. Effectiveness of Lactobacillus reuteri DSM 17938 for the prevention of nosocomial diarrhea in children: a randomized, double‐blind, placebo‐controlled trial. Pediatr Infect Dis J. 2016;35(2):142‐145. [DOI] [PubMed] [Google Scholar]
- 47. Szajewska H, Kotowska M, Mrukowicz JZ, Arma'nska M, Mikolajczyk W. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. J Pediatr. 2001;138(3):361‐365. [DOI] [PubMed] [Google Scholar]
- 48. Huang NN, Wang GZ, Wang JF, Yuan YX. Risk factors for neonatal nosocomial enteric infection and the effect of intervention with BIFICO. Eur Rev Med Pharmacol Sci. 2016;20(17):3713‐3719. [PubMed] [Google Scholar]
- 49. Honeycutt TCB, El Khashab M, Wardrop RM 3rd, et al. Probiotic administration and the incidence of nosocomial infection in pediatric intensive care: a randomized placebo‐controlled trial. Pediatr Crit Care Med. 2007;8(5):452‐458. [DOI] [PubMed] [Google Scholar]
- 50. Mastretta E, Longo P, Laccisaglia A, et al. Effect of Lactobacillus GG and breast‐feeding in the prevention of rotavirus nosocomial infection. J Pediatr Gastroenterol Nutr. 2002;35(4):527‐531. [DOI] [PubMed] [Google Scholar]
- 51. Bruzzese E, Fedele MC, Bruzzese D, et al. Randomised clinical trial: a Lactobacillus GG and micronutrient‐containing mixture is effective in reducing nosocomial infections in children, vs. placebo. Aliment Pharmacol Ther. 2016;44(6):568‐575. [DOI] [PubMed] [Google Scholar]
- 52. Wanke M, Szajewska H. Lack of an effect of Lactobacillus reuteri DSM 17938 in preventing nosocomial diarrhea in children: a randomized, double‐blind, placebo‐controlled trial. J Pediatr. 2012;161(1):40‐43. [DOI] [PubMed] [Google Scholar]
- 53. Hojsak I, Tokić Pivac V, Močić Pavić A, Pasini AM, Kolaček S. Bifidobacterium animalis subsp. lactis fails to prevent common infections in hospitalized children: a randomized, double‐blind, placebo‐controlled study. Am J Clin Nutr. 2015;101(3):680‐684. [DOI] [PubMed] [Google Scholar]
- 54. Salomão MCC, Heluany‐Filho MA, Menegueti MG, Kraker MEAD, Martinez R, Bellissimo‐Rodrigues F. A randomized clinical trial on the effectiveness of a symbiotic product to decolonize patients harboring multidrug‐resistant gram‐negative bacilli. Rev Soc Bras Med Trop. 2016;49(5):559‐566. [DOI] [PubMed] [Google Scholar]
- 55. Ljungquist O, Kampmann C, Resman F, Riesbeck K, Tham J. Probiotics for intestinal decolonization of ESBL‐producing Enterobacteriaceae: a randomized, placebo‐controlled clinical trial. Clin Microbiol Infect. 2020;26(4):456‐462. [DOI] [PubMed] [Google Scholar]
- 56. Kwon JH, Bommarito KM, Reske KA, et al. Randomized controlled trial to determine the impact of probiotic administration on colonization with multidrug‐resistant organisms in critically ill patients. Infect Control Hosp Epidemiol. 2015;36(12):1451‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tranberg A, Klarin B, Johansson J, Påhlman LI. Efficacy of Lactiplantibacillus plantarum 299 and 299v against nosocomial oropharyngeal pathogens in vitro and as an oral prophylactic treatment in a randomized, controlled clinical trial. MicrobiologyOpen. 2021;10(1):e1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ferrie S, Daley M. Lactobacillus GG as treatment for diarrhea during enteral feeding in critical illness: randomized controlled trial. J Parenter Enteral Nutr. 2011;35(1):43‐49. [DOI] [PubMed] [Google Scholar]
- 59. Sharma B, Srivastava S, Singh N, Sachdev V, Kapur S, Saraya A. Role of probiotics on gut permeability and endotoxemia in patients with acute pancreatitis: a double‐blind randomized controlled trial. J Clin Gastroenterol. 2011;45(5):442‐448. [DOI] [PubMed] [Google Scholar]
- 60. Fukushima Y, Miyaguchi S, Yamano T, et al. Improvement of nutritional status and incidence of infection in hospitalised, enterally fed elderly by feeding of fermented milk containing probiotic Lactobacillus johnsonii La1 (NCC533). Br J Nutr. 2007;98(5):969‐977. [DOI] [PubMed] [Google Scholar]
- 61. Litton E, Anstey M, Broadhurst D, et al. Early and sustained Lactobacillus plantarum probiotic therapy in critical illness: the randomised, placebo‐controlled, restoration of gut microflora in critical illness trial (ROCIT). Intensive Care Med. 2021;47(3):307‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mizuta M, Endo I, Yamamoto S, et al. Perioperative supplementation with bifidobacteria improves postoperative nutritional recovery, inflammatory response, and fecal microbiota in patients undergoing colorectal surgery: a prospective, randomized clinical trial. Biosci Microbiota Food Health. 2016;35(2):77‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu S, Hu P, Du X, Zhou T, Pei X. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta‐analysis of randomized, placebo‐controlled trials. Indian Pediatr. 2013;50(4):377‐381. [DOI] [PubMed] [Google Scholar]
- 64. Szajewska H, Wanke M, Patro B. Meta‐analysis: the effects of Lactobacillus rhamnosus GG supplementation for the prevention of healthcare‐associated diarrhoea in children. Aliment Pharmacol Ther. 2011;34(9):1079‐1087. [DOI] [PubMed] [Google Scholar]
- 65. Mantegazza C, Molinari P, D'Auria E, Sonnino M, Morelli L, Zuccotti GV. Probiotics and antibiotic‐associated diarrhea in children: a review and new evidence on Lactobacillus rhamnosus GG during and after antibiotic treatment. Pharmacol Res. 2018;128:63‐72. [DOI] [PubMed] [Google Scholar]
- 66. Szajewska H, Ruszczyński M, Radzikowski A. Probiotics in the prevention of antibiotic‐associated diarrhea in children: a meta‐analysis of randomized controlled trials. J Pediatr. 2006;149(3):367‐372. [DOI] [PubMed] [Google Scholar]
- 67. Liao W, Chen C, Wen T, Zhao Q. Probiotics for the prevention of antibiotic‐associated diarrhea in adults: a meta‐analysis of randomized placebo‐controlled trials. J Clin Gastroenterol. 2021;55(6):469‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile‐associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12(12):CD006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhao J, Li L, Chen C, Zhang G, Cui W, Tian B. Do probiotics help prevent ventilator‐associated pneumonia in critically ill patients? A systematic review with meta‐analysis. ERJ Open Res. 2021;7(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Batra P, Soni KD, Mathur P. Efficacy of probiotics in the prevention of VAP in critically ill ICU patients: an updated systematic review and meta‐analysis of randomized control trials. J Intensive Care. 2020;8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gu WJ, Wei CY, Yin RX. Lack of efficacy of probiotics in preventing ventilator‐associated pneumonia. Chest. 2012;142(4):859‐868. [DOI] [PubMed] [Google Scholar]
- 72. Wang J, Liu K, Ariani F, Tao L, Zhang J, Qu JM. Probiotics for preventing ventilator‐associated pneumonia: a systematic review and meta‐analysis of high‐quality randomized controlled trials. PLoS One. 2013;8(12):e83934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hojsak I. Probiotics in children: what is the evidence? Pediatr Gastroenterol Hepatol Nutr. 2017;20(3):139‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Olsen R, Greisen G, Schrøder M, Brok J. Prophylactic probiotics for preterm infants: a systematic review and meta‐analysis of observational studies. Neonatology. 2016;109(2):105‐112. [DOI] [PubMed] [Google Scholar]
- 75. AlFaleh K, Anabrees J. Efficacy and safety of probiotics in preterm infants. J Neonatal Perinatal Med. 2013;6(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 76. Newman AM, Arshad M. The role of probiotics, prebiotics and synbiotics in combating multidrug‐resistant organisms. Clin Ther. 2020;42(9):1637‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Szajewska H, Wanke M, Patro B. Meta‐analysis: the effects of Lactobacillus rhamnosus GG supplementation for the prevention of healthcare‐associated diarrhoea in children. Aliment Pharmacol Ther. 2011;34(9):1079‐1087. [DOI] [PubMed] [Google Scholar]
- 78. Kaila M, Arvilommi H, Soppi E, Laine S, Isolauri E. A prospective study of humoral immune responses to cow milk antigens in the first year of life. Pediatr Allergy Immunol. 1994;5(3):164‐169. [DOI] [PubMed] [Google Scholar]
- 79. Vernaya M, McAdam J, Hampton MD. Effectiveness of probiotics in reducing the incidence of Clostridium difficile‐associated diarrhea in elderly patients: a systematic review. JBI Database Syst Rev Implement Rep. 2017;15(1):140‐164. [DOI] [PubMed] [Google Scholar]
- 80. Jafarnejad S, Shab‐Bidar S, Speakman JR, Parastui K, Daneshi‐Maskooni M, Djafarian K. Probiotics reduce the risk of antibiotic‐associated diarrhea in adults (18–64 years) but not the elderly (>65 years): a meta‐analysis. Nutr Clin Pract. 2016;31(4):502‐513. [DOI] [PubMed] [Google Scholar]
- 81. Ale EC, Binetti AG. Role of probiotics, prebiotics, and synbiotics in the elderly: insights into their applications. Front Microbiol. 2021;12:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.


