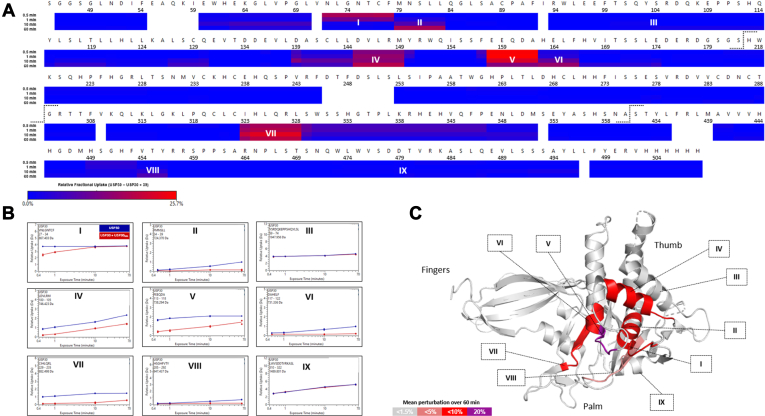
Fig. 4.
HDX-MS characterizes the conformational dynamics of USP30inhbinding to USP30.A, residue-level heat map indicating that USP30inh induces solvent protection in several regions of USP30. The plot displays the difference in relative fractional uptake between the holo- and apo-form of the protein over 1 h. Regions of that have the greatest perturbation following USP30inh binding are labeled regions I–IX. B, comparative uptake plots of regions I–IX for apo- and holo-USP30 states. C, integrated HDX-MS and X-ray crystal structure of USP30 in complex with di-Ub. Regions of perturbation between apo- and holo-USP30 states of HDX-MS data are colored according to magnitude of change. The data indicate that the USP30inh-binding interface is located between the USP30 thumb and palm domains of the protein. Numbering is in accordance with the crystal structure of 5OHK. Dotted lines indicate the site of cleavage and removal of unstable disordered sequences from the full-length USP30 protein. HDX-MS, Hydrogen–deuterium exchange mass spectrometry; Ub, ubiquitin; USP30, ubiquitin-specific protease 30.