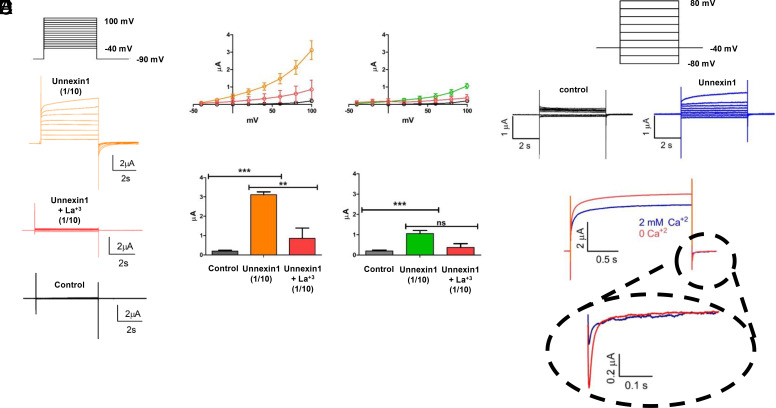
Fig. 3.
Unx1-mediated macroscopic currents are sensitive to voltage. Unx1-expressing oocytes were recorded using the cut-open oocyte technique (A–C) and two-electrode voltage clamp (D and E). (A) Representative traces of currents obtained with the voltage-pulse protocol depicted on the top from a batch of oocytes with negligible endogenous currents (control), injected with a 1/10 dilution of Unx1 mRNA before (orange) and after adding La3+ to a final concentration of 250 µM. (B) Current vs. voltage (IV) plots (Top) and maximal current (Bottom) obtained from the same batch of oocytes shown in A injected with 1/10 dilution of Unx1 mRNA in the absence (orange, n=13) and presence of 250 µM La3+ (red; n = 5; **P < 0.05 and ***P < 0.001). (C) As B from oocytes injected with a 1/100 dilution of Unx1 mRNA in the absence (green, n = 6) and presence of 250 µM La3+ (red; n = 3; ***P < 0.002). For comparison, we included measurements from uninjected oocytes (gray; n = 16) of the same batch. (D and E) Electrophysiological recordings of Unx1-expressing oocytes obtained by the two-electrode oocyte technique. Representative traces of currents were obtained with the holding protocol –40 mV from –80 mV at intervals of 20 mV to 80 mV from a batch of oocytes coinjected with Unx1 1/10 dilution of mRNA and anti-Cx38 antisense. (E) Reduction of Unx1 induced current by extracellular Ca2+. Representative traces in the absence (orange) and presence of 2 mM Ca2+ in the bath solution. In the presence of extracellular Ca2+ both, the outward and tail inward current evoked by a pulse to +80 mV and repolarization to –40 mV, respectively, were reduced by approximately 30% and 60%.