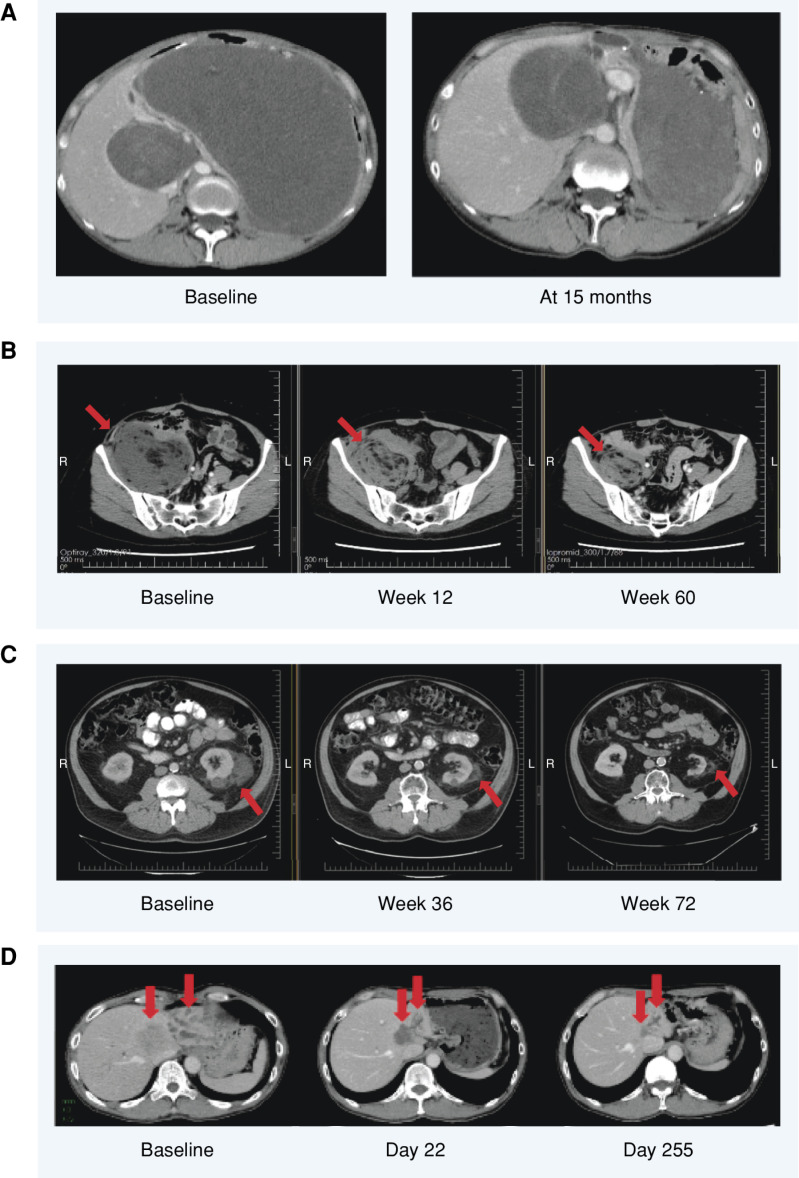
Figure 3.
Example patient scans showing responses and sustained disease stabilizations with brigimadlin. A, A 54-year-old female with a retroperitoneal MDM2-amplified WDLPS. The patient was heavily pretreated before being enrolled in the present trial, in which she received brigimadlin 20 mg q3w. The patient experienced a partial response (31% tumor reduction) starting at cycle 2. At cycle 10, the dose was reduced to 10 mg due to grade 2 neutropenia not recovering within 14 days. At cycle 19, a further tumor shrinkage (47% reduction) was observed. At cycle 29, the dose was further reduced to 5 mg due to grade 3 anemia. Treatment was finally discontinued after cycle 33 due to progressive disease. B, A 53-year-old female with a retroperitoneal MDM2-amplified WDLPS first diagnosed in July 2010 who, after four surgical resections, received doxorubicin for 6 cycles starting in March 2020 before progressing and being enrolled to the present trial. The patient received brigimadlin 80 mg D1q3w and experienced a partial response at cycle 3 (32% tumor reduction). The dose was reduced to 60 mg at cycle 4 due to neutropenia, to 50 mg at cycle 5 due to neutropenia, to 45 mg at cycle 8 due to thrombocytopenia, then to 30 mg at cycle 12 (December 2021) due to neutropenia, and finally to 20 mg at cycle 20 due to neutropenia. At data cutoff, the patient was ongoing in the study with a partial response (42% best tumor reduction, more than 25 months on treatment). C, A 65-year-old male with stage IV MDM2-amplified left perirenal DDLPS. Following previous treatment with adriamycin and olaratumab, followed by radiotherapy, the patient received brigimadlin 45 mg q3w. The patient achieved stable disease and stayed on treatment for nearly 2 years before experiencing progressive disease. D, A 51-year-old male with MDM2-amplified cholangiocarcinoma. Following 3 prior lines of therapy, he received brigimadlin 80 mg q3w, which was reduced to 45 mg q3w for 38 days in cycle 2 due to grade 4 thrombocytopenia and grade 4 neutropenia. The patient achieved a partial response by day 22, which was still evident on day 360; maximum tumor shrinkage was −73%. The patient remained on treatment for 13.3 months before experiencing progressive disease. B–D, The red arrows indicate the tumor sites. L, left; R, right.