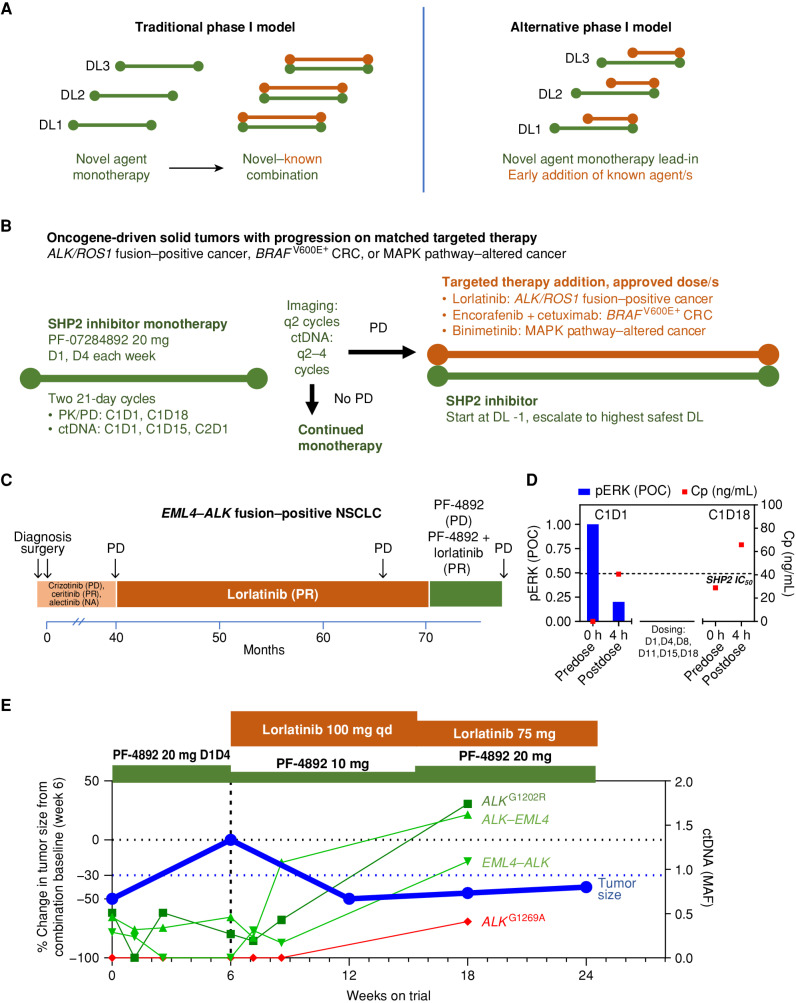
Figure 2.
Proof-of-concept clinical activity in an ALK fusion–positive NSCLC patient with resistance to multiple ALK inhibitors. A, Overview of traditional vs. alternative phase I combination trial design. Left, traditional phase I first-in-human trials require a new anticancer agent to be investigated as monotherapy, with the maximum tolerated dose/recommended dose for expansion identified, prior to allowing a combination with a second anticancer drug. If the investigational agent is ineffective on its own, treated patients do not have the opportunity to benefit from a potentially efficacious combination. Right, an alternative design allows patients to receive treatment with a potentially effective combination after an initial period of treatment with the study drug as monotherapy. B, Implementation of early combination testing strategy with the investigational SHP2 inhibitor PF-07284892. Prior to trial enrollment, eligible patients had experienced PD with appropriate targeted therapy. Patients begin treatment with PF-07284892 monotherapy on study. Early combination with appropriate targeted therapy (lorlatinib, encorafenib + cetuximab, or binimetinib, each at the approved dose) may be initiated after a minimum of 6 weeks of PF-07284892 monotherapy, in the absence of ongoing grade ≥3 toxicity or DLT, and after PD (symptoms of PD without tumor growth ≥20% was allowed). At the start of the combination, the PF-07284892 dose must be lowered if the monotherapy dose level the patient was enrolled to has not yet been cleared from a safety perspective. The dose may subsequently be escalated to the highest monotherapy dose that has been cleared. C, The patient's previous systemic therapies included four approved ALK inhibitors. Parentheses show the best overall response to each treatment. D, Peripheral blood was isolated from the patient prior to and 4 hours after dosing with PF-07284892 on C1D1 and C1D18, and levels of PF-07284892 in plasma and of pERK in ex vivo CSF1-stimulated peripheral blood monocytes were analyzed (C1D18 samples for pERK were not available). The last dose of PF-07284892 prior to the C1D18 predose sampling was C1D15. The horizontal dashed line indicates PF-07284892 concentration required to inhibit 50% of pERK in cells in vitro. E, Changes in the sum of the longest tumor diameters of target lesions (blue, normalized to the start of combination) and in EML4–ALK, ALK–EML4, ALKG1202R (shades of green), and ALKG1269A (red) in ctDNA. C, cycle; Cp, plasma concentration; CRC, colorectal cancer; ctDNA, circulating tumor DNA; D, day; DL, dose level; MAF, mean allele frequency; NA, not available; PD, progressive disease; pERK, phosphorylated ERK; PF-4892, PF-07284892; PK/PD, pharmacokinetics/pharmacodynamics; POC, percent of control; PR, partial response; qd, every day.