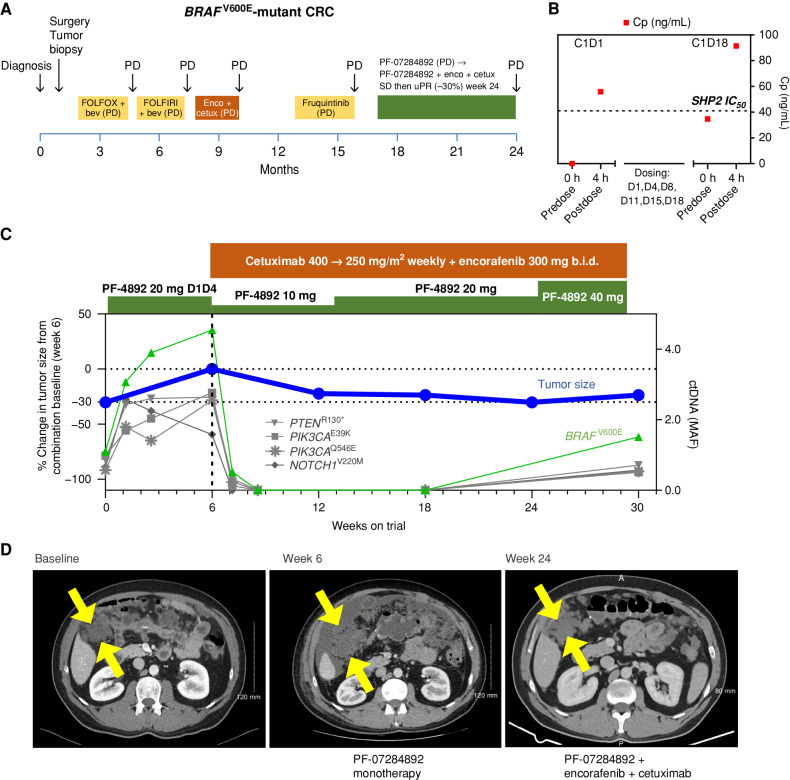
Figure 3.
PF-07284892 overcomes intrinsic resistance to encorafenib + cetuximab in a BRAFV600E-mutant CRC patient. A, The patient's previous systemic therapies were chemotherapy + bevacizumab, encorafenib + cetuximab, and fruquintinib, with the best overall response PD, indicating primary progression/intrinsic resistance to each therapy. B, Levels of PF-07284892 (red squares) in plasma, as in Fig. 2D; blood samples for pERK were not available. C, Change in the sum of the longest tumor diameters of target lesions and in BRAFV600E (and other mutations) in ctDNA over time, as in Fig. 2E. D, Imaging of a right-sided intra-abdominal target lesion mass during study treatment. The patient experienced one AE with monotherapy (grade 2 ascites not related to study treatment) and three grade 1 AEs with combination treatment (headache, fatigue, and acneiform rash, the latter a known toxicity of cetuximab). bev, bevacizumab; b.i.d., twice daily; C, cycle; cetux, cetuximab; Cp, plasma concentration; CRC, colorectal cancer; D, day; enco, encorafenib; FOLFIRI, folinic acid, fluorouracil, irinotecan; FOLFOX, folinic acid, fluorouracil, oxaliplatin; MAF, mean allele frequency; PD, progressive disease; PF-4892, PF-07284892; SD, stable disease; uPR, unconfirmed partial response.