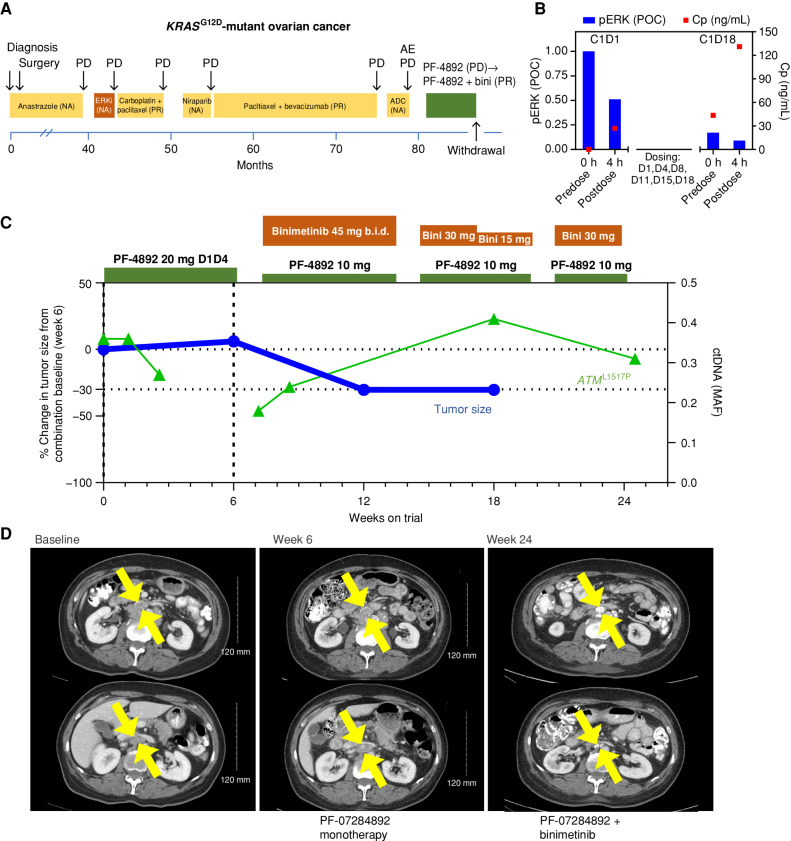
Figure 4.
PF-07284892 sensitizes a patient with KRASG12D-mutant ovarian cancer to the MAPK pathway inhibitor binimetinib. A, Prior treatments for metastatic disease included ASN-007 (investigational ERK inhibitor), chemotherapy, niraparib, and SGN-STNV (investigational antibody–drug conjugate). B, Levels of PF-07284892 in plasma (red squares) and pERK in ex vivo CSF1-stimulated peripheral blood monocytes (blue bars), as in Fig. 2D. C, Change in the sum of the longest tumor diameters of target lesions and of ATML1517P in ctDNA over time, as in Fig. 2E. D, Imaging of two abdominal target lesions during study treatment. All treatment-related AEs were grade 1 except for edema (grades 1–2, starting on monotherapy, worsening on the combination, and leading to dose modification), fatigue (grades 2–3), weight gain, diarrhea, and eczema (each grade 2 and resolved by the end of treatment). ADC, antibody–drug conjugate; b.i.d., twice daily; bini, binimetinib; C, cycle; Cp, plasma concentration; D, day; ERKi, ERK inhibitor; MAF, mean allele frequency; NA, not available; PD, progressive disease; PF-4892, PF-07284892; POC, percentage of control.