Fig. 1.
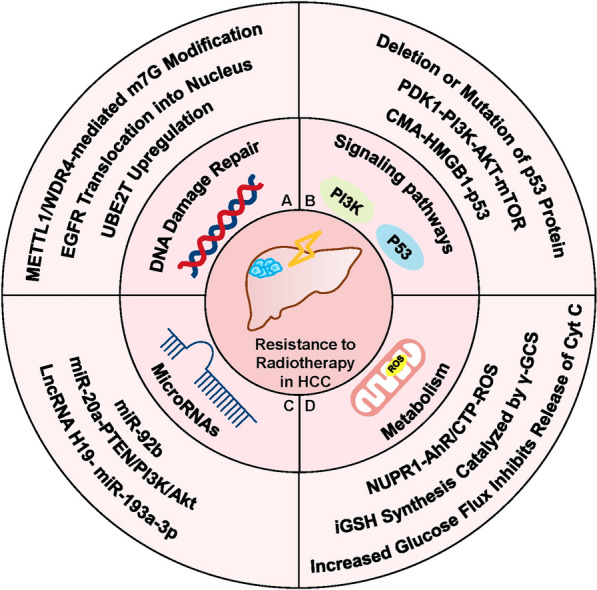
Molecular mechanisms of resistance to radiotherapy in HCC. A DNA damage repair is an important contributor to radiation resistance. Both METTL1/WDR4-mediated modification of m7G tRNA and EGFR mutations enhance the efficiency of DNA double-strand break (DSB) repair in the nonhomologous end-joining (NHEJ) pathway by augmenting the activity of the catalytic subunits of DNA-dependent protein kinases (DNA-PKcs). Upregulation of the ubiquitin-conjugating enzyme E2T (UBE2T) in HCC cells plays a similar role. B Changes in multiple signalling pathways in HCC cells affect their sensitivity to radiation. Deletion and mutation of p53 both decrease the radiosensitivity of HCC, and chaperone-mediated autophagy (CMA) impairs the efficacy of radiotherapy by downregulating p53. The effect of phosphoinositide-dependent protein kinase-1 (PDK1) on HCC radiosensitivity is mediated by activation of the PI3K/AKT/mTOR signalling pathway, which inhibits DNA damage repair. C MicroRNAs (miRNAs) are involved in the regulation of radiosensitivity in HCC by affecting cell proliferation and cell cycle. MiR-92b, mir-20a and miR-193a-3p confer radiation resistance to HCC cells in different ways. D The role of metabolism in radiosensitivity cannot be ignored. Glucose addiction in HCC cells promotes phospholipid synthesis, which inhibits cytochrome c release and reduces radiation-induced apoptosis. γ-Glutamylcysteine synthetase (γ-GCS) and nuclear protein 1 (NUPR1) inhibit cellular oxidative stress by producing GSH and modulating the AhR/CTP signalling axis, respectively, thereby enhancing cell viability after radiotherapy
