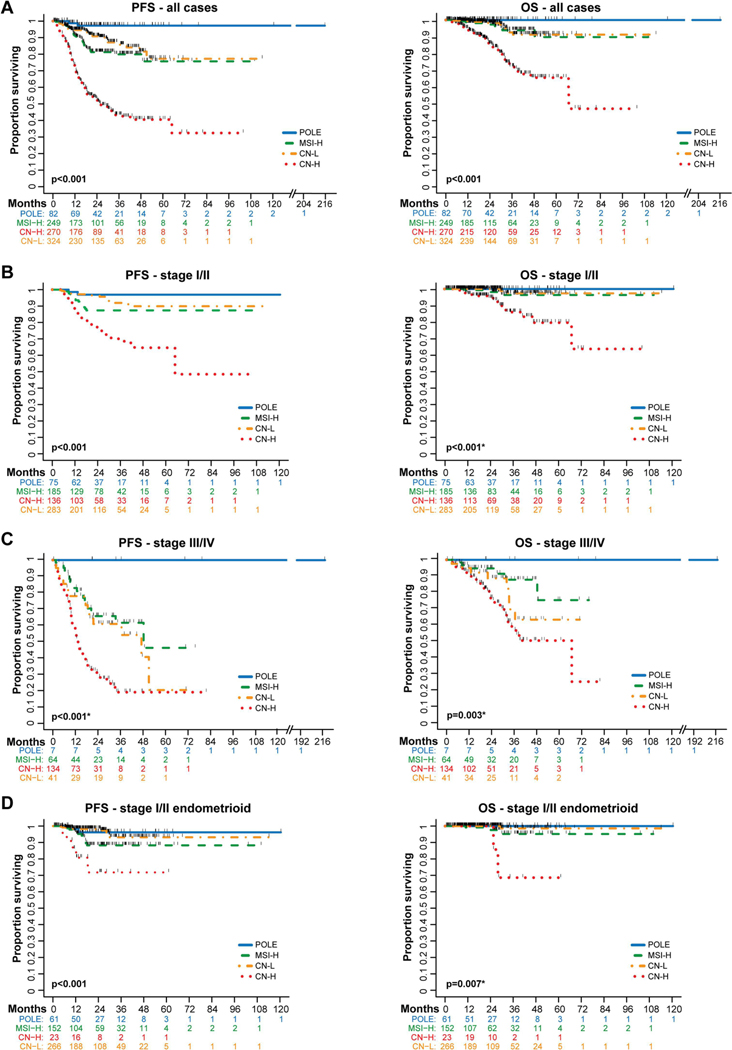
Figure 4. Survival outcomes of endometrial cancer patients by molecular subtype using an integrated clinical sequencing – immunohistochemistry-based classification approach.
Survival was assessed in 925 patients with endometrial cancer of all histologic types whose tumors were subjected to MSK-IMPACT prior to recurrence, had upfront surgery, were surgically staged, and had surgery performed at our institution (see Supplementary Fig. S1). A. Kaplan-Meier curve comparing progression-free survival (PFS) and overall survival (OS) in all 925 endometrial cancer patients by molecular subtype. B. PFS (n=679) and OS (n=502) of endometrial cancer patients with stage I/II disease. C. PFS and OS of endometrial cancer patients with stage III/IV disease (n=246). For stage III and stage IV analysis separately, see Supplementary Fig. S3. D. PFS and OS of patients with stage I/II endometrioid endometrial cancer (n=502). Survival compared with log-rank test. *p-value defined excluding the POLE molecular subtype due to the lack of events. CN-H, copy number-high; CN-L, copy number-low; MSI-H, microsatellite instability-high.