IMPORTANCE:
Outcomes of tracheostomized patients with COVID-19 are seldomly investigated with conflicting evidence from the existing literature.
OBJECTIVES:
To create a study evaluating the impact of COVID-19 on tracheostomized patients by comparing clinical outcomes and weaning parameters in COVID-19 positive and negative cohorts.
DESIGN, SETTING, AND PARTICIPANTS:
A retrospective observational cohort study of 604 tracheostomized patients hospitalized in 16 ICUs in New York City between March 9, 2020, and September 8, 2021.
MAIN OUTCOMES AND MEASURES:
Patients were stratified into two cohorts: 398 COVID-19 negative (COVID–ve) and 206 COVID-19 positive (COVID+ve) patients. Clinical characteristics, outcomes, and weaning parameters (first pressure support [PS], tracheostomy collar [TC], speech valve placement, and decannulation) were analyzed.
RESULTS:
COVID+ve had fewer comorbidities including coronary artery disease, congestive heart failure, malignancy, chronic kidney disease, liver disease, and HIV (p < 0.05). Higher Fio2 (53% vs 44%), positive end-expiratory pressure (PEEP) (7.15 vs 5.69), Pco2 (45.8 vs 38.2), and lower pH (7.41 vs 7.43) were observed at the time of tracheostomy in COVID+ve (p < 0.005). There was no statistical difference in post-tracheostomy complication rates. Longer time from intubation to tracheostomy (15.90 vs 13.60 d; p = 0.002), tracheostomy to first PS (2.87 vs 1.80 d; p = 0.005), and TC placement (11.07 vs 4.46 d; p < 0.001) were seen in COVID+ve. However, similar time to speech valve placement, decannulation, and significantly lower 1-year mortality (23.3% vs 36.7%; p = 0.001) with higher number of discharges to long-term acute care hospital (LTACH) (23.8% vs 13.6%; p = 0.015) were seen in COVID+ve.
CONCLUSIONS AND RELEVANCE:
Patients with COVID-19 required higher Fio2 and PEEP ventilatory support at the time of tracheostomy, with no observed change in complication rates. Despite longer initial weaning period with PS or TC, similar time to speech valve placement or decannulation with significantly lower mortality and higher LTACH discharges suggest favorable outcome in COVID-19 positive patients. Higher ventilatory support requirements and prolonged weaning should not be a deterrent to pursuing a tracheostomy.
Keywords: COVID-19, decannulation, tracheostomy, ventilator weaning, weaning parameters
KEY POINTS
Question: We studied the impact of COVID-19 on tracheostomized patients by analyzing clinical outcomes and weaning parameters in COVID-19 positive and negative cohorts.
Findings: This retrospective, observational, cohort study showed that COVID-19 patients required higher Fio2 and positive end-expiratory pressure ventilatory support at the time of tracheostomy, with no observed change in complication rates. Despite longer initial weaning period with pressure support or tracheostomy collar, similar time to speech valve placement or decannulation with significantly lower mortality and higher long-term acute care hospital discharges were observed.
Meaning: Higher ventilatory support and prolonged weaning should not be a deterrent to pursuing a tracheostomy.
In 2019, a novel coronavirus was identified in Wuhan, China, leading to the global pandemic of COVID-19 disease. The virus that causes COVID-19 is designated severe acute respiratory syndrome coronavirus 2. Compared with other viral infections such as influenza, increased rates of mechanical ventilation, and higher rates of mortality were reported in patients with COVID-19 (1). Increasing severity of acute respiratory distress syndrome (ARDS) in COVID-19 frequently leads to prolonged weaning (2).
Per 2001 guidelines by The American College of Chest Physicians, the readiness for Spontaneous Breathing Trials and ultimately the ability to be weaned off a ventilator includes the improvement of the cause of the respiratory failure, Pao2/Fio2 greater than or equal to 150 or oxygen saturation greater than or equal to 89% on Fio2 less than or equal to 40% and positive end-expiratory pressure (PEEP) less than or equal to 5, pH greater than 7.25 with little to no vasopressor support with hemodynamic stability and inspiratory drive (3). In patients not meeting the above criteria with projected prolonged wean, tracheostomies are recommended. Benefits of tracheostomies include less need for deep sedation, shorter weaning time, therefore, a shorter intensive care unit (ICU) and hospital stay (4). Early tracheostomy is typically defined as less than 14 days and is reported to shorten the duration of artificial ventilation and ICU stay, although does not significantly alter mortality (5). Percutaneous technique is usually the procedure of choice over open due to lower risks of surgical site infections and stomatitis (6–9). During the pandemic, this technique was also preferred for minimization of hypoxia and aerosolization (10).
Early literature on tracheostomized COVID-19 patients focused on risks of transmission to healthcare professionals. This was later followed by several reviews analyzing outcomes of weaning, decannulation, and survival, but conflicting evidence still exists surrounding the outcomes of tracheostomized COVID-19 patients. We present a unique cohort study comparing the clinical characteristics, outcomes, and weaning parameters of tracheostomized patients with and without COVID-19, with the aim to evaluate the clinical impact of COVID-19 and provide further guidance on management of tracheostomized patients.
METHODS
Study Design, Setting, and Population
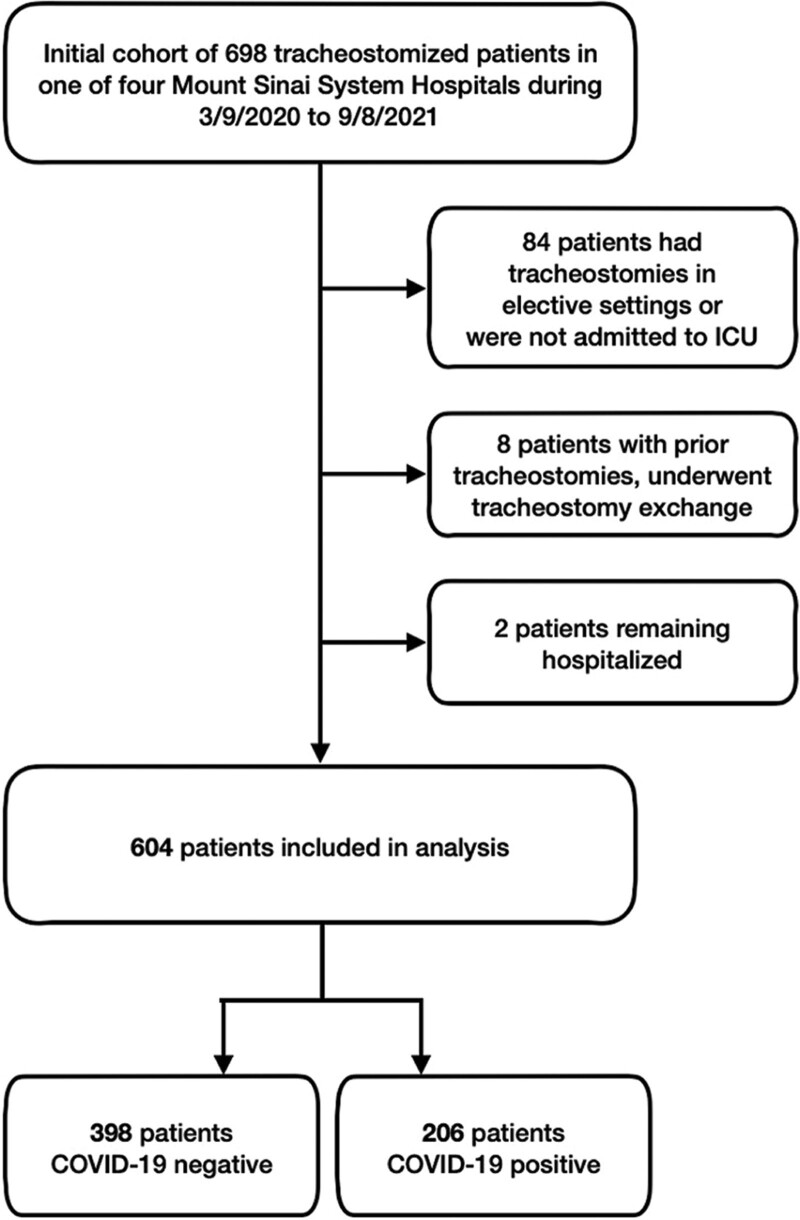
This retrospective, observational, cohort study included all consecutive tracheostomized adult patients (> 18 yr) hospitalized in 16 ICUs within the Mount Sinai Health System in New York City including the Mount Sinai Hospital, Mount Sinai Morningside, West, and Beth Israel, between March 9, 2020, and September 8, 2021. Exclusion criteria included patients who underwent a tracheostomy in an elective setting, had an existing tracheostomy requiring an exchange, and who remained hospitalized at the point of data collection. The selection process is demonstrated in Figure 1.
Figure 1.
A flow chart of patients included into the study.
A total of 604 patients were identified and included in the study. The diagnosis of COVID-19 was confirmed by reverse transcriptase-polymerase chain reaction of nasopharyngeal or oropharyngeal specimens. The institutional review board of Mount Sinai Health System initially approved this STUDY-21-01159 on August 17, 2021. As no direct patient contact or intervention from the study group was needed, informed consent was waived. Researchers exclusively used de-identified data. The procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975.
Data Collection
Clinical data was accessed via the electronic medical record system, Epic, and relevant de-identified data were extracted following review of patient medical charts. Patient demographics, coexisting medical conditions, and clinical data including medications, oxygen requirements, vital signs, and laboratory data were collected. Coexisting medical conditions and presenting symptoms were obtained from physician documentation. One-year mortality was gathered via Epic chart review.
Statistical Analysis
All analyses were performed with R software (Version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria). Continuous variables are presented as means and sds for normally distributed data or as medians and interquartile ranges for nonparametric data. Categorical variables are summarized as frequencies and percentages. Differences in distributions of characteristics of those with and without COVID-19 were examined using Student t test for continuous variables and chi-square test or Fisher exact test (for samples with < 15 observations) for categorical variables. p values were calculated with the use of two-sided exact tests and p value of less than or equal to 0.05 was considered to indicate statistical significance.
RESULTS
Baseline Characteristics
A total of 604 patients were identified and included in the study. Patients were stratified into two cohorts: 398 patients were COVID-19 negative (COVID–ve) and 206 patients were COVID-19 positive (COVID+ve). The primary reason for admission to ICU for COVID+ve was respiratory failure. Indications of tracheostomy in COVID–ve included pneumonia (n = 66, 16.6%), ARDS (n = 45, 11.3%), other pulmonary disease (n = 32, 8.0%), neurologic disease (neuromuscular/neurovascular disease including anoxic brain injury from cardiac arrest) (n = 176, 44.2%), and other causes (n = 79, 19.8%).
The baseline characteristics of both groups are summarized in Table 1. Both groups had a similar mean age (62.08 vs 61.55 yr) and gender distribution (35.7% vs 36.4% females). A larger Hispanic population was seen in COVID+ve (9.5% vs 18.4%). COVID+ve had fewer comorbidities including coronary artery disease, congestive heart failure, malignancy, chronic kidney disease, liver disease, and HIV (p < 0.05).
TABLE 1.
Comparison of Clinical Characteristics of Tracheostomized Patients With and Without COVID-19
| Characteristics | COVID-19 Negative (n = 398) | COVID-19 Positive (n = 206) | p |
|---|---|---|---|
| Demographics | |||
| Age, yr, mean (sd) | 62.08 (14.84) | 61.55 (13.16) | 0.665 |
| Females | 142 (35.7) | 75 (36.4) | 0.93 |
| Body mass index, kg/m2, mean (sd) | 26.40 (9.06) | 27.93 (7.93) | 0.042 |
| Race | < 0.001 | ||
| White | 124 (31.2) | 61 (29.6) | |
| Hispanic | 38 (9.5) | 38 (18.4) | |
| African-American | 102 (25.6) | 29 (14.1) | |
| Asian | 26 (6.5) | 6 (2.9) | |
| Other | 108 (27.1) | 72 (35.0) | |
| Comorbidities | |||
| Hypertension | 210 (52.8) | 113 (54.9) | 0.687 |
| Hyperlipidemia | 115 (28.9) | 64 (31.1) | 0.645 |
| Diabetes | 130 (32.7) | 79 (38.3) | 0.193 |
| Coronary artery disease | 102 (25.6) | 29 (14.1) | 0.002 |
| Congestive heart failure | 76 (19.1) | 13 (6.3) | < 0.001 |
| Chronic obstructive pulmonary disease/asthma | 44 (11.1) | 34 (16.5) | 0.078 |
| Interstitial lung disease | 5 (1.3) | 4 (1.9) | 0.499 |
| Other lung disease | 31 (7.8) | 10 (4.9) | 0.229 |
| Connective tissue disease | 10 (2.5) | 2 (1.0) | 0.237 |
| Malignancy | 61 (15.3) | 19 (9.2) | 0.049 |
| Chronic kidney disease | 62 (15.6) | 17 (8.3) | 0.016 |
| End-stage renal disease | 39 (9.8) | 11 (5.3) | 0.063 |
| Liver disease | 48 (12.1) | 10 (4.9) | 0.004 |
| HIV | 16 (4.0) | 2 (1.0) | 0.042 |
| Cerebrovascular accident | 43 (10.8) | 16 (7.8) | 0.295 |
| Dementia | 18 (4.5) | 8 (3.9) | 0.834 |
Note—except where indicated, data are number of patients, with percentages in parentheses. Continuous variables are presented as means and sds for normally distributed data. Categorical variables are summarized as frequencies and percentages. Differences in distributions of characteristics of those with and those without COVID-19 were analyzed using Student t test for continuous variables and χ2 test or Fisher exact test (for samples with < 15 observations) for categorical variables. Boldface values indicate statistical significance (p < 0.05).
Ventilator Settings at the Time of Tracheostomy
As demonstrated in Table 2, the mean time from intubation to tracheostomy for COVID+ve was longer with 15.90 days, compared with that of COVID–ve of 13.60 days (p = 0.002). The mean time from last day of continuous sedation to tracheostomy was shorter in COVID+ve with 0.95 versus 3.05 days in COVID–ve (p < 0.001). More sedative agents were used in COVID+ve compared with COVID–ve (4.39 vs 3.22; p < 0.001). The mean Fio2 and PEEP at the time of tracheostomy for COVID+ve was 53% and 7.15, compared with 44% and 5.69 for COVID–ve (p < 0.001). The mean tidal volume was statistically similar in both groups (412.40 mL in COVID–ve, 399.24 mL in COVID+ve; p = 0.093). The mean pH and Pco2 at the time of tracheostomy for COVID+ve was 7.41 and 45.8, compared with 7.43 and 38.2 for COVID–ve (p = 0.004, p < 0.001, respectively).
TABLE 2.
Comparison of ICU Therapies in Patients With and Without COVID-19
| Characteristics | COVID-19 Negative (n = 398) | COVID-19 Positive (n = 206) | p |
|---|---|---|---|
| Time from intubation to tracheostomy (d) | 13.60 (8.66) | 15.90 (8.60) | 0.002 |
| Time from continuous sedation to tracheostomy (d) | 3.05 (5.23) | 0.95 (2.69) | < 0.001 |
| Time from antibiotics to tracheostomy (d) | 1.97 (3.71) | 2.45 (4.92) | 0.185 |
| Number of sedative agents since admission, mean (sd) | 3.22 (1.23) | 4.39 (1.18) | < 0.001 |
| Ventilator settings, mean (sd) | |||
| Fio2 at the time of tracheostomy (%) | 44.24 (12.96) | 53.28 (16.79) | < 0.001 |
| Positive end-expiratory pressure at the time of tracheostomy (mm Hg) | 5.69 (1.87) | 7.15 (2.71) | < 0.001 |
| Tidal volume at the time of tracheostomy (mL) | 412.40 (93.51) | 399.24 (83.38) | 0.093 |
| Blood gas, mean (sd) | |||
| Potential of hydrogen at the time of tracheostomy | 7.43 (0.07) | 7.41 (0.07) | 0.004 |
| Pco2 at the time of tracheostomy | 38.17 (10.24) | 45.81 (13.14) | < 0.001 |
Note—except where indicated, data are number of patients, with percentages in parentheses. Continuous variables are presented as means and sds for normally distributed data. Differences in distributions of characteristics of those with and those without COVID-19 were analyzed using Student t test. Boldface values indicate statistical significance (p < 0.05).
Clinical Outcomes and Weaning Parameters
Time from tracheostomy to first pressure support (PS) (2.87 vs 1.80 d; p = 0.005) and tracheostomy collar (TC) placement (11.07 vs 4.46 d; p < 0.001) were longer in COVID+ve. However, there was no statistically significant difference in the days to first speech valve placement (23.09 vs 29.91 d; p = 0.062) and decannulation post-tracheostomy (49.2 vs 54.40 d; p = 0.474) between the two groups. Similar proportion of patients were eventually decannulated during the hospital stay: 28.1% in COVID–ve (n = 112), 30.6% in COVID+ve (n = 63; p = 0.594). Hospital mortality rates were similar in both groups: 36.4% (n = 145) versus 35.9% (n = 74; p = 0.973). One-year mortality was significantly lower in COVID+ve of 23.3% (n = 48) compared with COVID–ve of 36.7% (n = 146) with p value of 0.001. More COVID–ve were discharged to a skilled nursing facility or a rehabilitation center (41.2% vs 32.5%), whereas more COVID+ve were discharged to a long-term acute care hospital (LTACH) (23.8% vs 13.6%) (p = 0.015).
Last, there was no statistical difference in the rate of complications post-tracheostomy between COVID–ve and COVID+ve. One hundred twenty-nine patients (21%) in total had complications post-tracheostomy across the cohorts: pneumonia (n = 40, 31%), tracheitis (n = 37, 28.7%), minor bleeding (n = 25, 19.4%), major bleeding requiring transfusion (n = 13, 10.1%), stenosis (n = 7, 5.4%), and other (n = 7, 5.4%). Most tracheostomies were performed percutaneously (n = 525, 86.9%). While many tracheostomies in COVID–ve were performed by an ICU team, more tracheostomies were performed by other specialties including general surgery and cardiothoracic surgery for COVID+ve.
There was a statistically significant decrease in number of complications associated with percutaneous tracheostomies (p < 0.01) and with tracheostomies by ICU team (p < 0.005) on regression analyses. There were no statistically significant associations between ventilator settings, recent sedation, antibiotics, and steroid use prior to the tracheostomy with the rate of complications.
Comparison of outcomes and weaning parameters are summarized in Table 3. Tracheostomy characteristics are summarized in Supplementary Data Table 1 (http://links.lww.com/CCX/B227).
TABLE 3.
Comparison of Clinical Outcomes and Weaning Parameters in Tracheostomized Patients With and Without COVID-19
| Outcomes | COVID-19 Negative (n = 398) | COVID-19 Positive (n = 206) | p |
|---|---|---|---|
| Ventilation weaning | |||
| Time from tracheostomy to first pressure support placement (d) | 1.80 (3.43) | 2.87 (5.28) | 0.005 |
| Time from tracheostomy to first trach collar placement (d) | 4.46 (5.87) | 11.07 (14.29) | < 0.001 |
| Time from tracheostomy to speech valve placement (d) | 23.09 (21.84) | 29.91 (22.08) | 0.062 |
| Time from tracheostomy to decannulation (d) | 49.32 (43.70) | 54.50 (46.11) | 0.474 |
| Eventual decannulation | 112 (28.1) | 63 (30.6) | 0.594 |
| LOS, d | |||
| Hospital LOS | 60.14 (56.40) | 54.29 (38.61) | 0.182 |
| ICU LOS | 40.22 (46.42) | 39.20 (24.27) | 0.769 |
| Mortality | |||
| Hospital mortality | 145 (36.4) | 74 (35.9) | 0.973 |
| One-yr mortality | 146 (36.7) | 48 (23.3) | 0.001 |
| Disposition | |||
| Home | 23 (5.8) | 14 (6.8) | 0.015 |
| Skilled nursing facility/rehabilitation | 164 (41.2) | 67 (32.5) | |
| Long-term acute care hospital | 54 (13.6) | 49 (23.8) | |
| Expired | 145 (36.4) | 73 (35.4) | |
| Others | 12 (3.0) | 3 (1.5) | |
| Complications from tracheostomy | 0.695 | ||
| Yes | 65 (16.3) | 37 (18.0) | |
| No | 333 (83.7) | 169 (82.0) | |
LOS = length of stay.
Note—except where indicated, data are number of patients, with percentages in parentheses. Continuous variables are presented as means and sds for normally distributed data. Categorical variables are summarized as frequencies and percentages. Differences in distributions of characteristics of those with and those without COVID-19 were analyzed using Student t test for continuous variables and χ2 test or Fisher exact test (for samples with < 15 observations) for categorical variables. Boldface values indicate statistical significance (p < 0.05).
DISCUSSION
Tracheostomy is one of the most frequently performed surgical procedures in the critically ill patient, with known benefits of reduction in days of mechanical ventilation, ICU, and hospital stays (11). The optimal timing and technique of tracheostomy have remained controversial even before the COVID-19 era. Some reported no impact of these on mortality and time to decannulation, whereas others claimed that an early tracheostomy helps reduce ventilator dependence and length of stay (LOS), but with lower 30-day survival (12–14).
Increased rates of mechanical ventilation and increased severity of ARDS with prolonged weaning have been reported to be associated with COVID-19 (1, 2). A higher survival rate in tracheostomized COVID-19 cohort when compared with the nontracheostomized has been described (15). Our large cohort study of tracheostomized COVID-19 and non-COVID-19 patients focused on comparison of clinical settings at the time of tracheostomy, clinical outcomes, and weaning parameters following the tracheostomy.
Clinical Settings: The Timing of Tracheostomy
Optimal timing of tracheostomy has remained controversial, even before the COVID-19 era. An average of 16.5 days from intubation to tracheostomy have been described in two reviews involving 47 studies (n = 5,268) and 37 studies (n = 3,876) of tracheostomized COVID-19 positive patients (12, 13). Owing to the nature of the disease of slow recovery and longer ventilator dependency of COVID ARDS, early tracheostomy was not an option for many due to inability to tolerate a loss of positive airway pressure during the tracheostomy procedure (16). A recent multicenter study of 549 patients describes association of early tracheostomies (defined as < 14 d from intubation) with shorter duration of ventilation and ICU stay (14, 17–19) but also with increased mortality (20, 21). Controversially, a study argues that an early tracheostomy is noninferior to late tracheostomy with improvement in LOS with no increased infections in clinicians (22).
Our study reports longer time from intubation to tracheostomy of 15.90 days in COVID+ve compared with 13.60 days of COVID–ve (p = 0.002). This can be explained by longer duration to medical stabilization in COVID+ve, earlier tracheostomy in non-COVID neurologic patients, and the availability of staffs and resources due to overburden on the health system during the pandemic.
Clinical Settings: Ventilator Settings
We report that tracheostomies were performed in COVID+ve when they were sicker with lower pH (p = 0.004), higher Pco2, and required higher ventilator settings than COVID–ve (p < 0.001). Our finding can be explained in two ways. ARDS is described in 42% of COVID+ve patients, with 61–81% of those requiring intensive care (23, 24). ARDS typically involves diffuse alveolar damage with increased epithelial barrier permeability, leading to reduced compliance, compromise of gas exchange and eventual hypoxemia (25). However, COVID ARDS differs from typical ARDS in its severity, longer onset time of the disease (8–12 d), and relatively normal lung compliance in some patients (26). Near-normal lung compliance and severe hypoxemia due to ventilation/perfusion mismatch is described in type-L patients of COVID ARDS, as opposed to more serious hypoxemia in the setting of low compliance in type-H patients, latter resembling classic ARDS (27). Such patients with good compliance may have been able to undergo the tracheostomy on high ventilator settings. Patients with COVID ARDS are reported to require higher ventilator settings such as PEEP, Pao2, and paralytic agents (2, 24). A review of 26 studies analyzing COVID-19 patients showed average PEEP ranging from 9 to 16.5 cm H2O, which was higher than mean PEEP of our study of 7.15 cm H2O (2), in contrast to 5.69 cm H2O of COVID–ve.
Second, it is also possible that more providers were willing to perform tracheostomy with higher ventilator settings than usual as they witnessed COVID+ve patients’ prolonged ventilator requirements and slow recovery.
Significantly longer duration of continuous sedation and greater use of sedative agents (p < 0.001) observed in ventilated COVID+ve patients are consistent with the current literature. Moderate to deep level of sedation is often needed to achieve ventilator synchrony in severe COVID-19 pneumonia and ARDS (28, 29). However, it should be recognized that prolonged and high sedation requirement by COVID+ patients not only creates pressure on the supply chain resulting in shortages of critical medications but also increases the rates of delirium, LOS, and mortality (28, 30).
Clinical Outcomes and Weaning Parameters
Longer time to first PS and TC placement were observed in COVID+ve, compared with COVID–ve (2.87 vs 1.80 d; p = 0.005 and 11.07 vs 4.46 d; p < 0.001, respectively). These findings were expected as COVID+ve were sicker with higher ventilator settings during tracheostomy.
Despite later timing of tracheostomy and higher ventilator settings during tracheostomy; hence, delayed PS placement for COVID+ve, both groups had similar recovery path thereafter with no significant difference in the timing of placement of speech valves and decannulation of tracheostomies. Eventually, both groups had similar ICU and hospital LOS.
Hospital mortality was also similar in both groups during the hospitalization but COVID+ve had better outcome upon discharge with significantly lower 1-year mortality compared with COVID–ve (23.3% vs 36.7%; p < 0.001). The lower 1-year mortality rate we deduced demonstrates initial clinical vulnerability and slow improvement of COVID+ve patients during an early to mid-phase of the disease process, followed by relatively stable and rapid recovery in late phase. It should be noted, however, that the 1-year mortality rates were obtained via chart review and not all deaths may have been recorded.
Furthermore, more tracheostomized COVID+ve were able to be discharged to a LTACH compared with COVID–ve (23.8% vs 13.6%; p = 0.015). This finding shows that COVID+ve were able to demonstrate their potential of ventilator weaning and tolerability of aggressive rehabilitation at the time of discharge (31). As a post-acute care facility, the care at LTACHs is driven by patients’ continued acute medical needs with its focus on facilitation of functional recovery and optimization of respiratory status, including liberation from prolonged mechanical ventilation (32). LTACHs have been proved to be an optimal facility for post-ICU care during COVID-19 pandemic with the rate of successful wean of 70.9% from prolonged mechanical ventilation (33, 34). The overall eventual favorable outcome in COVID+ve patients can be explained by the observed healthier population with less baseline comorbidities as well.
Our study reports no difference in the rate and type of complications between the two cohorts (Table 3). Tracheostomies performed by the ICU team were equally as safe as those performed by others (Supplementary Data Table 1, http://links.lww.com/CCX/B227). Tracheostomies in patients with difficult anatomy are typically deferred to surgical specialties, which likely explains the higher rates of post-operative complications by these specialties.
Limitations
Despite the patients coming from a unique large urban population, given the variability inpatient demographics in the region, our findings can be considered generalizable. However, several limitations are acknowledged.
Our article included a cohort of patients undergoing tracheostomies of different etiologies including nonpulmonary indications with the aim to determine the effect of COVID-19. Direct comparisons between COVID+ve and COVID–ve with ARDS were not performed as many COVID+ve ARDS patients did not survive or were too unstable to undergo a tracheostomy. We acknowledge that comparing patients who underwent a tracheostomy due to severe respiratory failure to those with a neurologic injury could limit valid comparability between the cohorts. This also likely explains the difference in comorbidities of the patients. However, this approach allowed our unique comparison study of a large cohort which we believe can provide useful information to clinicians when managing tracheostomized patients.
Our study as a retrospective, observational study was exposed to possible confounding and selection bias. Furthermore, this study was performed during the peak of the pandemic, which consisted of different strains of COVID-19 with unvaccinated cohort. Further studies need to be performed for applicability to the newer COVID-19 strains and vaccinated population.
CONCLUSIONS
Patients with COVID-19 required higher Fio2 and PEEP ventilatory support at the time of tracheostomy, with no observed change in complication rates. Although initial weaning period with PS or TC were longer, similar time to speech valve placement or decannulation with significantly lower mortality and higher discharges to LTACH suggest favorable outcome in COVID-19 positive patients. Higher ventilatory support and prolonged weaning should not be a deterrent to pursuing a tracheostomy.
Supplementary Material
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
Dr. Bahk was involved in conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, visualization,and reviewing and editing the original draft and writing. Drs. Dolan and Sharma were involved in data curation, investigation, reviewing and editing the original draft and writing. Dr. Sehmbhi was involved in formal analysis, methodology, and validation. Drs. Fung and Lee were involved in conceptualization, project administration, resources, supervision, validation, and reviewing and editing the writing.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Cobb NL, Sathe NA, Duan KI, et al. : Comparison of clinical features and outcomes in critically ill patients hospitalized with COVID-19 versus influenza. Ann Am Thorac Soc 2021; 18:632–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Cattaneo E, Florio G, et al. : Mechanical ventilation parameters in critically ill COVID-19 patients: A scoping review. Crit Care 2021; 25:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacIntyre NR, Cook DJ, Ely EW, et al. ; American College of Chest Physicians: Evidence-based guidelines for weaning and discontinuing ventilatory support: A collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Medicine. Chest 2001; 120:375S–395S [DOI] [PubMed] [Google Scholar]
- 4.Durbin CG: Tracheostomy: Why, when, and how? Respir Care 2010; 55:1056–1068 [PubMed] [Google Scholar]
- 5.Griffiths J, Barber VS, Morgan L, et al. : Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ 2005; 330:1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung NH, Napolitano LM: Tracheostomy: Epidemiology, indications, timing, technique, and outcomes. Respir Care 2014; 59:895–915; discussion 916–919 [DOI] [PubMed] [Google Scholar]
- 7.Brass P, Hellmich M, Ladra A, et al. : Percutaneous techniques versus surgical techniques for tracheostomy. Cochrane Database Syst Rev 2016; 7:CD008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Bussy S, Mahajan B, Folch E, et al. : Tracheostomy tube placement: Early and late complications. J Bronchol Interv Pulmonol 2015; 22:357–364 [DOI] [PubMed] [Google Scholar]
- 9.Delaney A, Bagshaw SM, Nalos M: Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: A systematic review and meta-analysis. Crit Care 2006; 10:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun BJ, Wolff CJ, Bechtold HM, et al. : Modified percutaneous tracheostomy in patients with COVID-19. Trauma Surg Acute Care Open 2020; 5:e000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durbin CG: Indications for and timing of tracheostomy. Respir Care 2005; 50:483–487 [PubMed] [Google Scholar]
- 12.Battaglini D, Premraj L, White N, et al. : Tracheostomy outcomes in critically ill patients with COVID-19: A systematic review, meta-analysis, and meta-regression. Br J Anaesth 2022; 129:679–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferro A, Kotecha S, Auzinger G, et al. : Systematic review and meta-analysis of tracheostomy outcomes in COVID-19 patients. Br J Oral Maxillofac Surg 2021; 59:1013–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrell Shreckengost CS, Foianini JE, Moron Encinas KM, et al. : Outcomes of early versus late tracheostomy in patients with COVID-19: A multinational cohort study. Crit Care Explor 2022; 4:e0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molin N, Myers K, Soliman AMS, et al. : COVID-19 tracheostomy outcomes. Otolaryngol Head Neck Surg 2022; 167:923–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schultz MJ, Pattnaik R, Dondorp AM: Walking the line between benefit and harm from tracheostomy in COVID-19. Lancet Respir Med 2020; 8:656–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avilés-Jurado FX, Prieto-Alhambra D, González-Sánchez N, et al. : Timing, complications, and safety of tracheotomy in critically ill patients with COVID-19. JAMA Otolaryngol Head Neck Surg 2020; 147:41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breik O, Nankivell P, Sharma N, et al. : Safety and 30-day outcomes of tracheostomy for COVID-19: A prospective observational cohort study. Br J Anaesth 2020; 125:872–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson A, Sunnergren O, Hammarskjöld A, et al. : Characteristics, complications, and a comparison between early and late tracheostomy: A retrospective observational study on tracheostomy in patients with COVID-19-related acute respiratory distress syndrome. Health Sci Rep 2022; 5:e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volo T, Stritoni P, Battel I, et al. : Elective tracheostomy during COVID-19 outbreak: To whom, when, how? Early experience from Venice, Italy. Eur Arch Otorhinolaryngol 2021; 278:781–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Wu Y, Zhu F, et al. : Tracheostomy in 80 COVID-19 patients: A multicenter, retrospective, observational study. Front Med (Lausanne) 2020; 7:994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwak PE, Connors JR, Benedict PA, et al. : Early outcomes from early tracheostomy for patients with COVID-19. JAMA Otolaryngol Head Neck Surg 2021; 147:239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C, Chen X, Cai Y, et al. : Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson PG, Qin L, Puah SH: COVID-19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust 2020; 213:54–56.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gosangi B, Rubinowitz AN, Irugu D, et al. : COVID-19 ARDS: A review of imaging features and overview of mechanical ventilation and its complications. Emerg Radiol 2022; 29:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Ma X: Acute respiratory failure in COVID-19: Is it “typical” ARDS? Crit Care 2020; 24:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattinoni L, Chiumello D, Rossi S: COVID-19 pneumonia: ARDS or not? Crit Care 2020; 24:154–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ammar MA, Sacha GL, Welch SC, et al. : Sedation, analgesia, and paralysis in COVID-19 patients in the setting of drug shortages. J Intensive Care Med 2020; 36:157–174 [DOI] [PubMed] [Google Scholar]
- 29.Flinspach AN, Booke H, Zacharowski K, et al. : High sedation needs of critically ill COVID-19 ARDS patients-a monocentric observational study. PLoS One 2021; 16:e0253778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasulo FA, Badenes R, Longhitano Y, et al. : Excessive sedation as a risk factor for delirium: A comparison between two cohorts of ARDS critically ill patients with and without COVID-19. Life (Basel) 2022; 12:2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demiralp B, Koenig L, Xu J, et al. : Time spent in prior hospital stay and outcomes for ventilator patients in long-term acute care hospitals. BMC Pulm Med 2021; 21:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller T, Canfield C, Buckingham T, et al. : Long-term acute care: Where does it fit in the health care continuum? Am J Crit Care 2016; 25:364–367 [DOI] [PubMed] [Google Scholar]
- 33.Grigonis AM, Mathews KS, Benka-Coker WO, et al. : Long-term acute care hospitals extend ICU capacity for COVID-19 response and recovery. Chest 2021; 159:1894–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saad M, Laghi FA, Brofman J, et al. : Long-term acute care hospital outcomes of mechanically ventilated patients with coronavirus disease 2019*. Crit Care Med 2022; 50:256–263 [DOI] [PMC free article] [PubMed] [Google Scholar]