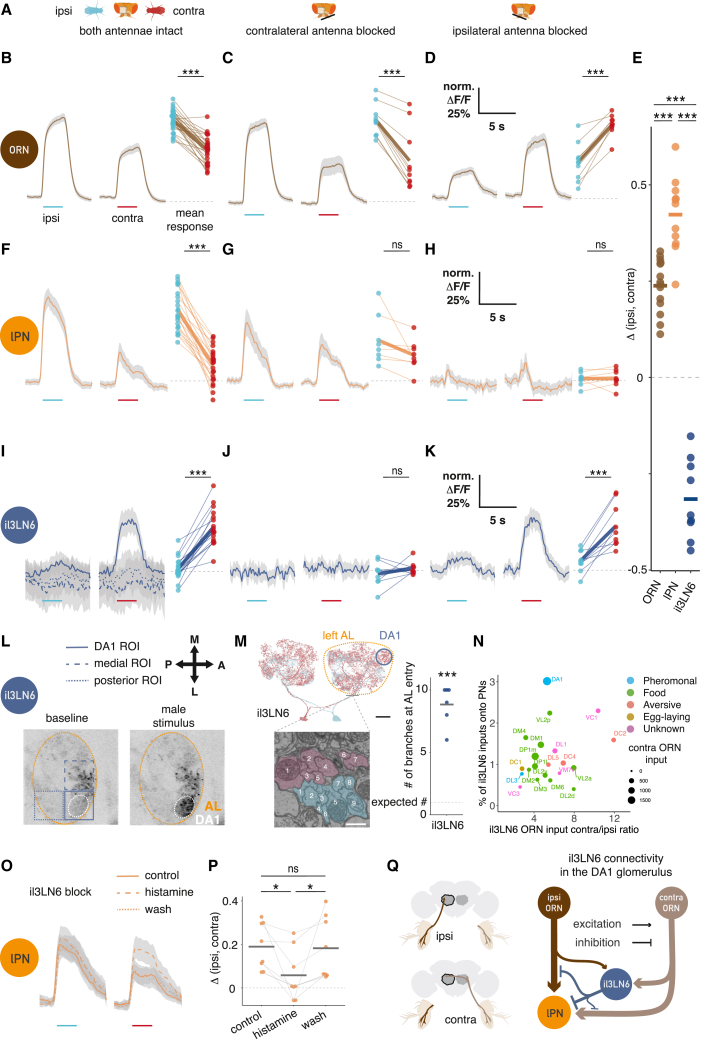
Figure 4.
An active mechanism increases bilateral contrast in cVA sensing
(A) Antennal manipulations and male presentation with respect to an imaging ROI (gray square).
(B–D) ORNs respond stronger to a male presented ipsilaterally. Antennae: both intact (B), contralateral blocked (C), and ipsilateral blocked (D). Left: GCaMP6f responses in ORN axons to ipsi- and contralateral male presentation. Male presentation time: cyan (ipsilateral) and red (contralateral) lines. Average response from 14 (28 hemispheres);10;10 flies, 6 trials, and gray area is SEM of biological replicates. Right: mean responses of hemispheres to ipsi- and contralateral stimuli.
(E) Bilateral contrast, calculated as the difference of mean responses to ipsi- and contralateral male presentation, in different cell types.
(F–H) lPNs respond stronger to a male presented ipsilaterally. Antennae: both intact (F), contralateral blocked (G), and ipsilateral blocked (H). Left: GCaMP6f responses in lPN axons to ipsi- and contralateral male presentation. Male presentation time marked by cyan (ipsilateral) and red (contralateral) lines. Average response from 11 (22 hemispheres);8;8 flies, 6 trials, and gray area is SEM of biological replicates. Right: mean responses of hemispheres to ipsi- and contralateral stimuli.
(I–K) il3LN6 responds stronger to a male presented contralaterally in the DA1 glomerulus and shows no responses in adjacent arbors. Antennae: both intact (I); contralateral blocked, only DA1 ROI shown (J); and ipsilateral blocked, only DA1 ROI shown (K). Left: GCaMP6f responses in lPN axons to ipsi- and contralateral male presentation. Male presentation time marked by cyan (ipsilateral) and red (contralateral) lines. Average response from 9 (18 hemispheres);9;9 flies, 6 trials, and gray area is SEM of biological replicates. Right: mean responses of hemispheres to ipsi- and contralateral stimuli.
(L) Example images of il3LN6 GAL4 (VT046100) GCaMP before and during contralateral male presentation (dorsal AL). Three ROIs were used to quantify the responses in different parts of il3LN6 (I). Pixel intensity represents GCaMP fluorescence.
(M) Left top: EM morphology of il3LN6 neurons in FAFB, partial reconstruction. Scale bars, 20 μm. Left bottom: axon cross sections of two il3LN6 neurons before entering the AL. Scale bars, 750 nm. Right: number of branches entering the AL for il3LN6 neurons from the hemibrain (2) and FAFB (4) datasets. Horizontal bar shows the mean (8.83 ± 1.46), which is significantly different from 1, the median for fly neurons.
(N) Connectivity of il3LN6 by glomerulus in the hemibrain dataset. x axis shows the ratio of contralateral and ipsilateral ORN input to il3LN6, y axis shows the fraction of il3LN6 inputs to uniglomerular PNs, and the size of the points is proportional to the number of contralateral ORN inputs to il3LN6 in a glomerulus. Five glomeruli with known bilateral ORN innervation (VA1d, VA1v, DC3, VL1, and VP1d) were excluded from this analysis due to missing ORN side information in the hemibrain.
(O) Blocking il3LN6 decreases bilateral contrast in lPN axons. Same stimulus as in (B), before (control), during (histamine), and after (wash) chemogenetic block of il3LN6 neurons, n = 8, 6 trials, and gray area is SEM of biological replicates.
(P) Quantification of O.
(Q) Connectivity of DA1 ORNs, lPNs, and il3LN6 in one hemisphere, data from hemibrain. The line width is proportional to the base 2 logarithm of the number of synapses for a given connection. Number of synaptic connections: ipsiORN-lPN: 5,184; contraORN-lPN: 3,580; ipsiORN-il3LN6: 359; ipsiORN-il3LN6: 1,901; il3LN6-lPN: 465; il3LN6-ipsiORN: 297; il3LN6-contraORN: 165.
See also Figure S4.