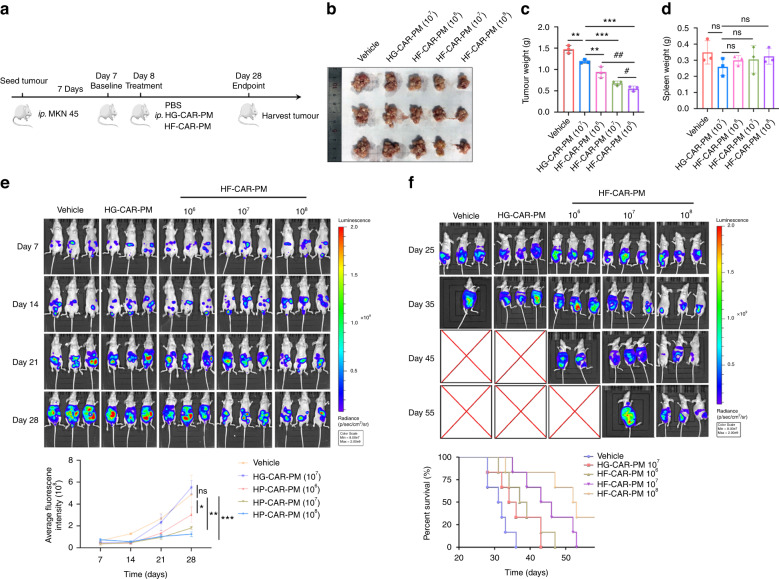
Fig. 4. The effect of CAR-modified PMs on tumour growth in vitro.
a The schematic representation of the experimental design. Male BALB/c nude mice (6–8 weeks old) were injected intraperitoneally with 1 × 106 MKN45-Luc cells/each, and seven days later groups were treated with PBS, 1 × 107 cells/100ul per mouse HG-CAR-PMs cells; low-dose 1 × 106, medium-dose 1 × 107 and high-dose 1 × 108 HF-CAR-PMs cells/100 μL per mouse for the HF-CAR-PMs treatment groups. b, c Representative image and quantitative analysis of tumour weight in the vehicle, HG-CAR-PMs, and HF-CAR-PMs treatment groups. d Quantitative analysis of spleen weight for the vehicle, HG-CAR-PMs, and HF-CAR-PMs treatment groups. e Quantitative analysis of fluorescence intensity captured every 7 days for each group. Tumour-bearing mice were injected intraperitoneally with 2 mg of luciferase substrate solution each (Fluorescence intensity range 8 × 107–2 × 109). f Representative bioluminescence imaging and survival curve of each treatment group. Statistical analysis by one-way ANOVA with Tukey’s multiple comparison test. *P < 0.05, **P < 0.01 and ***P < 0.001.