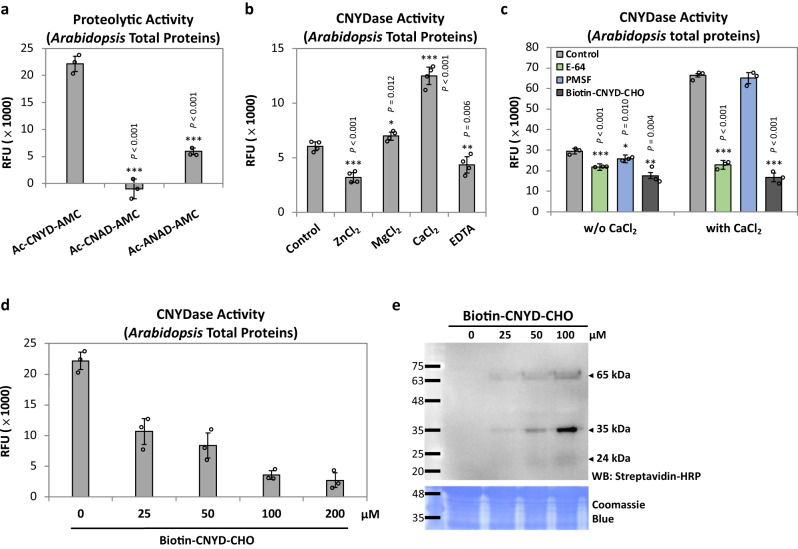
Fig. 4. Proteolytic specificity of CNYD motif and the properties of CNYD-targeted protease in Arabidopsis.
a Examination of the proteolytic activity of Arabidopsis protein extract using three fluorogenic protease substrates (CNYD, CNAD, and ANAD). b CNYDase activity of Arabidopsis protein extract supplemented with buffer only (control), ZnCl2, MgCl2 or CaCl2, or EDTA. c CNYDase activity of Arabidopsis protein extract supplemented with buffer only (control), PMSF, E-64, or biotin–CNYD–CHO under the condition with or without CaCl2 for 1 h prior to substrate incubation. d CNYDase activity and e gel blot of biotinylated proteins from Arabidopsis protein extract incubated with different concentrations of biotin–CNYD–CHO. Biotinylated proteins were detected by western blotting with streptavidin–HRP, and Coomassie Blue staining shows total protein loaded. a, b Relative fluorescent units (RFU) of the cleaved fluorophores were measured after 5 h incubation with the substrate. c, d RFU were measured after 10 h incubation with the substrate. Each assay was performed by incubating 50 µg protein extract from untreated Arabidopsis with 25 µM substrate. Values are means ± SD of three biological replicates. Each replicate was obtained from pooled tissues of three plants. P values were calculated by one-tailed unpaired t-test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).