Table 4.
Comparison between the proposed and reported MLC methods for determination of drugs under study
| Method | Proposed method | Reported method [14] | Reported method [18] | Reported method [19] | Reported method [20] |
|---|---|---|---|---|---|
| Technique |
MLC, C18, HPLC–UV |
Bonded b-CD column, HPLC–UV |
HPLC–UV Hyper ODS2, Column C18 |
HPTLC | Micellar electrokinetic capillary electrophoretic |
| Organic modifier | Totally free | Tetraethylammonium acetate | Mixture of 45 mL Acetonitrile and 55 mL of water | Acetone‒ethanol‒ethyl acetate‒2% sodium dodecyl sulfate‒glacial acetic acid (3:2:4:1:0.5, V/V) |
0.02 M monobasic sodium phosphate Adjusted to pH 3.0 (with 40% phosphoric acid) and acetonitrile) |
| Analytes similarity |
AMP, SLB CFP, CFX |
AMP, CFP, SLB | CFX, SLB | CFP, SLB | AMP, SLB |
| Runtime | 6 min | 28.3 min | 3.6 | 5 min | 20 min |
| GAPI assessment | 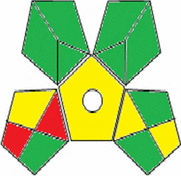 |
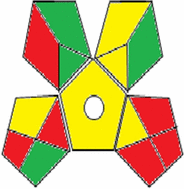 |
 |
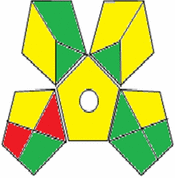 |
 |
| AGREE |  |
 |
 |
 |
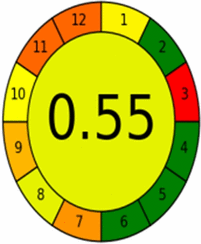 |
