Abstract
Purpose
This study presents a comparison of pupil changes according to cataract grade between low-energy femtosecond laser-assisted cataract surgery (FLACS) and conventional phacoemulsification (CP) in the same patient.
Patients and Methods
Data from surgical records from patients submitted to cataract surgery with CP in one eye and FLACS in the other were retrospectively reviewed. The inclusion criterion was both eyes of the same patient having the same cataract grade in accordance with Lens Opacity Classification System (LOCS) III. Total pupil variation (TPV) was measured after recorded images, with intraindividual comparison between techniques, according to cataract grade (≤3 and >3), age and cumulative dissipated energy (CDE).
Results
The study included a total of 124 eyes of 62 patients (mean age 72.65 ± 7.64 years). Analysis showed a statistically significant difference in TPV between techniques in the grade ≤3 cataract group (0.08 ± 0.22 mm²; p=0.034), with less pupil narrowing with FLACS, but not in the grade >3 group (0.01 ± 0.23 mm²; p=0.849). Regarding CDE, a significant difference (p<0.001) was found between techniques in both softer and harder cataracts, with lower values for FLACS. Correlation between CDE and TPV was significant for CP (p=0.021) but not for FLACS (p=0.922). TPV was significantly lower in older patients (age >74 years) for both techniques (p<0.001).
Conclusion
There was a statistically significant difference between techniques (although of mild clinical relevance), with less reduction of pupil area with FLACS in softer cataracts (grade ≤3), as compared to CP. Higher levels of CDE are associated with more pupil narrowing in CP.
Keywords: cataract grade, pupil, low energy FLACS, femtosecond laser, phacoemulsification
Introduction
Pupil size is of paramount importance in cataract surgery. Although considered a safe and efficient technique,1,2 femtosecond laser-assisted cataract surgery (FLACS) has been associated with intraoperative miosis as opposed to conventional phacoemulsification (CP).3,4 This counterback is prone to increase complication rates of the several surgical steps of cataract surgery, with posterior capsule rupture as a paramount risk. Intraoperative miosis is reported in most publications involving FLACS, regardless of the pattern of photo disruption pulse.5–7 Femtosecond lasers currently produce two different patterns: low-frequency (kHz) high-energy pulses (µJ) and high-frequency (MHz) low-energy pulses (nJ).8 Most of the published evidence concerns high-energy and low-frequency devices, not contemplating low-energy and high-frequency devices at all; or, very seldom, studies present global results without differentiating between the two types of pulse.9,10
More recently, some published studies point to a nearly absent laser-induced miosis with low energy FLACS.11–13 The explanation for this feature is liable to reside in the fact that a “low-energy” pulse is associated with smaller laser spots and lower energy delivered per pulse, thus reducing the impact on the neighbour tissues and resulting in lower levels of prostaglandins; in the end, this would reflect on minor or absent intraoperative pupil narrowing. Moreover, cataract grade is known to have an impact on energy parameters during phacoemulsification,14 such as cumulative dispersed energy (CDE). Cumulative dissipated energy (CDE) is described as the amount of ultrasound energy employed to remove a cataractous lens15 and it is defined as the product of the total phacoemulsification time (in minutes) and the average phacoemulsification power (%) divided by 100.
Our present study aims to compare intraindividually (within-subject) pupil area variation between “low-energy” FLACS and CP according to cataract degree.
Materials and Methods
The charts of all patients submitted to cataract surgery with standard phacoemulsification in one eye and femtosecond laser–assisted surgery in the contralateral eye, between April 2015 and October 2020, at Hospital da Luz Arrabida (HLA) were reviewed. The choice of the technique (FLACS or CP) was based solely on the availability (as it rotates between hospitals) of the femtosecond platform: if available, FLACS was performed, otherwise CP was the employed technique. The surgical devices employed were the LDV Z8 femtosecond laser platform (Ziemer Ophthalmic Systems AG, Port, Switzerland) and the Centurion Vision (Alcon Laboratories, Inc., Fort Worth, TX, USA) phacoemulsification system.
The present retrospective study received the approval of the Ethics Committee at Hospital da Luz Arrabida (HLA), Porto, and complied with the tenets of the “Declaration of Helsinki”, all patients having signed a consent form regarding their medical records’ review.
Inclusion and Exclusion Criteria
The inclusion criterion was both eyes of the same patient having the same cataract degree in accordance with the Lens Opacity Classification System (LOCS) III. Exclusion criteria were ocular surgery besides cataract surgery, history of trauma, corneal scarring or pathology, abnormal iris, glaucoma, pseudoexfoliation syndrome or any other ocular comorbidity. Exclusion criteria also included significant axial anisometropia (a difference equal to or greater than 1 mm in axial length between eyes of same patient).
Chart Review and Data Collection
Clinical and video recording data were collected after chart review of surgeries performed between April 2015 and October 2020. The Lens Opacities Classification System (LOCS) III was chosen for cataract grading, upon lens evaluation at the slit lamp. Biometry, corneal topography and tomography were performed with a combined optical interferometer and Placido–dual Scheimpflug device (Galilei G6, Ziemer Ophthalmic Systems AG, Port, Switzerland). Macular spectral-domain optical coherence tomography was performed with an ophthalmic imaging platform (Carl Zeiss, Oberkochen, Germany).
Regarding pupil measurement, images were taken from recorded surgical videos at two time points: at the beginning of both CP and FLACS (just before docking) and at the end of the surgical procedure, for both techniques. In the FLACS group, additional pupil measurement from images taken after docking release (femtosecond laser administered) was performed.
Cataract subgroups were built following a cut-off value of 3, with a cataract grade ≤3 subgroup (soft/medium cataracts) and another subgroup with >3 (harder cataracts), according to LOCS III classification. Each scale on LOCS III is a decimalized scale ranging from 0.1 (a completely clear or colorless lens) to 5.9 (upper value on the cortical opacity (C) and posterior subcapsular opacity (P) scales, indicating complete opacification of the cortex or posterior capsule) and 6.9 on the nuclear opacity (NO) and nuclear color (NC) scales (indicating advanced opacification and brunescence of the nucleus). The final grade’s score is obtained by summing up the score in each parameter (scale).
Surgical Technique
Two comprehensive surgeons performed all surgeries (R.S., A.M.) and adopted the same surgical protocol. Phacoemulsification was accomplished by using the Active Fluidics™ torsional phacoemulsification device (Centurion Vision, Alcon Laboratories, Inc.), ensuring irrigation, emulsification, and aspiration of lens material. The femtosecond laser machine employed in all FLACS was the portable LDV Z8 (Ziemer, Inc.), and it was used to realize capsulotomy, lens fragmentation and corneal incisions. All operations were unilateral, for both CP and FLACS techniques. Phacoemulsification sequentially followed laser in the FLACS patients, with no patient mobilization; these subsequent surgical steps, were accomplished equally in both groups (FLACS and CP).
The preoperative protocol was universal, consisting of placement of an ophthalmic insert containing tropicamide and phenylephrine hydrochloride, in the inferior conjunctival fornix, along with oxybuprocaine hydrochloride eye drops 20 minutes before the surgical intervention. No patient received any anti-inflammatory or miosis prevention drug preoperatively or intraoperatively (such as intracameral mydriatic agents); being widely integrated in surgical protocols in most centers elsewhere, this specific absence was explained to each patient, with respective given consent. Intracameral cefuroxime was universally administered for endophthalmitis prevention.
The laser parameters employed in the FLACS group were as follows: 100% laser energy for lens fragmentation (6-mm diameter radial pattern with six sectors) and 90% laser energy for capsulotomy (5.2-mm diameter) and corneal incisions (2.8 mm and 1.2 mm, respectively, main and secondary).
Pupil Area Measurement
In order to measure the pupil area, images were obtained from surgical videos at the protocol time points. The adopted imaging software was ImageJ (Fiji, version 2.0.0-rc-49/1.51a), as in previous studies,11,12 which converts pixels into millimeters and allows straightforward area measurements using the pupil margin as a boundary marker. For calibration purposes, the constant limbus horizontal diameter (as automatically provided by Galilei) was used.
Statistical Analysis
Analysis aimed at the assessment of pupil area changes between the beginning and the end of surgery, ie, the total pupil variation (TPV),12,13 comparing FLACS with CP. Intraindividual and interindividual subgroup analysis were performed to evaluate the variation in pupil area regarding cataract degree and age.
Regarding comparison of the total pupil variation (TPV) between FLACS and CP in the same patient (within-subject), a 2-tailed paired t-test was performed. Pupil size variation among subgroups subjected to the same surgery technique and cumulative dissipated energy (CDE) differences were tested using the same methodology (2-tailed t-test). Due to non-normality, all tests were validated with their non-parametric equivalent. A mixed-design analysis of variance (ANOVA) was employed in order to compare differences in pupil area between groups. For that matter, cataract grade (≤3 or >3) and age (≤ or > the median age) were considered between-subjects factors. On the other hand, type of surgical technique (FLACS or CP) was considered a within-subjects factor.
Differences in CDE between groups were also compared using a mixed-design ANOVA with type of surgery as a within-subjects factor and cataract grade as a between-subjects factor. Mauchly’s Test was used to assess sphericity assumption before the analysis; in the cases of no assumption compliance, the Greenhouse–Geisser correction was performed. A correlation between total pupil variation (TPV) and CDE was tested for both FLACS and CP. The Pearson correlation test was used, and the results were confirmed with a non-parametric test (Spearman correlation test). Correlations between TPV and surgery duration, and between CDE and surgery duration were also tested using the same methodology.
A significance level of α=0.05 and a power of 95% was set for this study; a p-value of <0.05 was regarded as statistically significant. The statistical analysis software was R (v. 4.1.2) in RStudio (v. 2022.07.0+548), and JMP (v. 16.0).
Results
Preoperative Patient Data
A total of 124 eyes of 62 patients had cataract surgery, with the same cataract degree in both eyes for each patient. The population consisted of 45 female (73%) and 17 male (27%) patients, with a mean age of 72.65 ± 7.64 years (median: 74, range: 59–95). Femtosecond laser-assisted surgery was performed in 62 eyes and conventional phacoemulsification was performed in the other 62 eyes; every patient had FLACS in one eye and CP in the other. No unexpected intraoperative events (such as IFIS, capsule related or other) were registered.
The study population was divided according to cataract grade using the Lens Opacity Classification System III (LOCS III), with 34 patients (68 eyes) having grade ≤3 in both eyes and 28 patients (56 eyes) having grade >3. The comparison of patients’ preoperative anatomical features and surgical total time (including FLACS laser phase) duration between techniques, for each group of cataract grade, is shown in Table 1. No statistically significant difference was registered between CP and FLACS preoperatively in each group.
Table 1.
Patient Preoperative Anatomical Data and Surgical Time Duration
| Cataract Grade (LOCS III) | Parameters | Mean ± SD | p-value | |
|---|---|---|---|---|
| FLACS | CP | |||
| ≤3 (n=34) | AL (mm) | 24.08 ± 1.69 | 24.02 ± 1.64 | 0.793 |
| ACD (mm) | 3.12 ± 0.42 | 3.14 ± 0.46 | 0.782 | |
| Time duration (min) | 13.14 ± 0.90 | 13.14 ± 0.84 | 0.990 | |
| >3 (n=28) | AL (mm) | 23.96 ±1.89 | 23.99 ± 1.84 | 0.806 |
| ACD (mm) | 2.98 ± 0.48 | 2.99 ± 0.46 | 0.899 | |
| Time duration (min) | 14.27 ± 0.91 | 14.24 ± 0.93 | 0.635 | |
Abbreviations: ACD, anterior chamber depth; AL, axial length; LOCS III, Lens Opacity Classification System III; SD, standard deviation.
Postoperative Data
In the group of patients submitted to FLACS, pupil area was measured at pre (beginning of surgery) and post laser treatment time points, with a mean change in pupil area of 0.01 ± 0.06 mm² for grade ≤3 cataract group and 0.01 ± 0.04 mm² for grade >3 cataract group; no statistically significant difference between them was found (p = 0.851).
Preoperative mean pupil area was 45.77 ± 7.69 mm² in the FLACS group and 45.78 ± 7.69 mm² in the CP group (p=0.414, paired t-test). Pupil area was assessed at the main protocol time points (at the beginning and the end of surgery) for both groups and the respective change (total pupil variation) was registered. Considering cataract grade ≤3, initial mean pupil area was 45.04 ± 6.96 mm² in the FLACS group and 45.04 ± 6.96 mm² in the CP group (p=0.999), whereas for cataract grade >3, preoperative mean pupil area was 46.65 ± 8.55 mm² in the FLACS group and 46.67 ± 8.53 mm² in the CP group (p=0.992).
The mean difference between techniques in total pupil variation (TPV) was 0.08 ± 0.22 mm² in the softer (grade ≤3) cataract group, and it was 0.01 ± 0.23 mm² in the harder (>3) cataract subgroup. A paired t-test showed a statistically significant difference in total pupil variation (TPV) between techniques in the subgroup of eyes with cataract degree ≤3 group (95% CI: 0.01 to 0.16; p=0.034), with less pupil narrowing with FLACS, but not in the harder (>3) cataract group (95% CI: −0.08 to 0.10; p=0.849). In the group of eyes with cataract degree >3, no statistically significant difference was registered (95% CI: −0.08 to 0.10; p=0.849), between techniques (Table 2).
Table 2.
Total Pupil Variation (mm²) versus Cataract Grade
| Cataract Grade LOCS III | TPV FLACS Mean ± SD | TPV CP Mean ± SD | Mean Diff. ± SD [95% CI] | Paired t-test (p-value) | Mixed Design ANOVA |
|---|---|---|---|---|---|
| ≤3 (n=34) | −5.77 ± 1.40 | −5.85 ± 1.40 | 0.08 ± 0.22 [0.01;0.16] | 0.034* | F1,60 = 2.60 0.112 |
| >3 (n=28) | −6.53 ± 1.57 | −6.53 ± 1.65 | 0.01 ± 0.23 [−0.08;0.10] | 0.849 | |
| Mean Diff. ± SD [95% CI] | 0.77 ± 3.01 [0.00;1.53] | 0.68 ± 3.08 [−0.11;1.46] | |||
| t-test (p-value) | 0.049* | 0.089 |
Note: *Statistically significant.
Abbreviations: LOCS III, Lens Opacity Classification System III; Mean Diff., mean difference; n, number of patients; SD, standard deviation.
Regarding within-technique analysis, a statistically significant difference in total pupil variation (TPV) was found between the cataract grade subgroups in FLACS, but not in CP. The mean difference between softer (grade ≤3) and harder (>3) cataract groups was 0.77 ± 3.0 mm² (95% CI: 0.00 to 1.53; p=0.049) in the FLACS group and it was 0.68 ± 3.08 mm² (95% CI: −0.11 to 1.46; p=0.089) in the CP group.
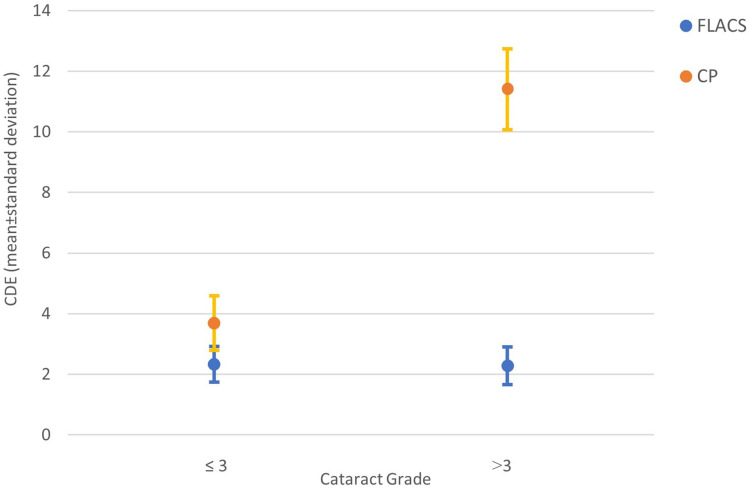
When comparing the cumulative dissipated energy (CDE) in each cataract grade subgroup, a statistically significant difference (p<0.001) was found between techniques in both softer and harder cataracts; namely with CDE mean values of 2.33 ± 0.58 with FLACS and 3.69 ± 0.89 with CP in the softer cataract subgroup, and 2.28 ± 0.62 and 11.41 ± 1.34, respectively with FLACS and CP in the harder cataract subgroup. The analysis within each technique revealed a statistically significant CDE mean difference of 7.72 ± 2.37 between cataract grade subgroups with CP (95% CI: 7.11 to 8.33; p<0.001), associated to higher CDE values in the harder cataract’s subgroup. On the contrary, no statistically significant difference in CDE was registered with FLACS and cataract grade subgroups, with a CDE mean difference of 0.04 ± 1.23 (95% CI: −0.26 to 0.36; p=0.754) (Figure 1).
Figure 1.
Mean ± standard deviation of CDE by cataract grade, for CP and FLACS.
Abbreviations: CDE, Cumulative Dissipated Energy; FLACS, femtosecond laser assisted cataract surgery; CP, conventional phacoemulsification.
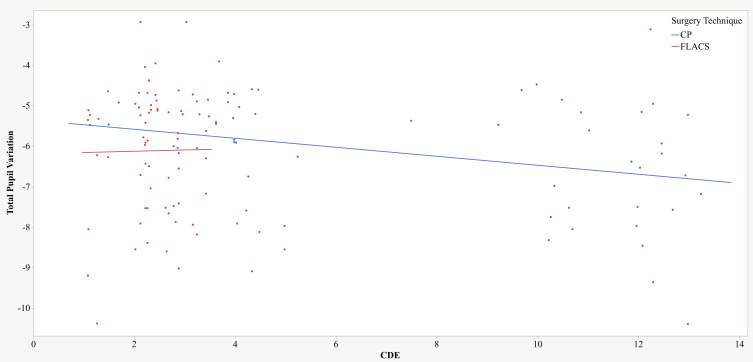
Correlation between total pupil variation (TPV) and cumulative dissipated energy (CDE) was tested for both techniques. In the FLACS group, no correlation was found between the TPV and CDE (Pearson’s r=0.01; p=0.922; R2=0.00), whereas in the CP group, a weak negative but statistically significant correlation was found (Pearson’s r=−0.29; p=0.021; R2=0.09), meaning that the higher the CDE values, the more negative the TPV (more pupil narrowing) (Figure 2).
Figure 2.
Relationship between Total Pupil Variation and Cumulative Dissipated Energy (CDE). Results separated by surgical technique: CP (blue) and FLACS (red).
Abbreviations: CDE, Cumulative Dissipated Energy; FLACS, femtosecond laser assisted cataract surgery; CP, conventional phacoemulsification.
Considering total pupil variation and duration of surgery, correlation (Pearson’s test) was expectedly significant for both techniques, with r=−0.50 and R2=0.25 (p<0.001) in the FLACS group and r=−0.46 and R2=0.21 (p<0.001) in the CP group.
Regarding age, two subgroups were built, below and above the median age (73 years). Preoperative mean pupil area was 40.53 ± 4.31 mm² in the FLACS group and 40.55 ± 4.31 mm² in the CP group (p=0.985) in the older (age >73 years) subgroup, while initial mean pupil area was 51.01 ± 6.71 mm² in the FLACS group and 51.01 ± 6.71 mm² in the CP group (p=0.999) in the younger (age ≤73 years) subgroup.
Regarding TPV, there was no statistically significant difference between techniques in either age subgroup. However, a statistically significant and similar difference occurred between age subgroups within each technique, with less pupil narrowing in the older (age >73 years) patients’ subgroup as compared to the younger (age ≤73 years); for both techniques the p-value was <0.001 (Table 3).
Table 3.
Total Pupil Variation (mm²) versus Age
| Age | TPV FLACS Mean ± SD | TPV CP Mean ± SD | Mean Diff. ±SD [95% CI] | Paired t-test (p-value) | Mixed Design ANOVA (p-value) |
|---|---|---|---|---|---|
| ≤73 (n=31) | −6.81 ± 1.52 | −6.84 ± 1.51 | 0.03 ± 0.14 [−0.02;0.08] | 0.246 | F1,60 = 0.17 0.682 |
| >73 (n=31) | −5.41± 1.52 | −5.47 ± 1.51 | 0.05 ± 0.29 [−0.05;0.16] | 0.306 | |
| Mean Diff. ± SD [95% CI] | 1.40 ± 2.71 [0.71;2.09] | 1.38 ± 2.75 [0.68;2.07] | |||
| t-test (p-value) | <0.001* | <0.001* |
Note: *Statistically significant.
Abbreviations: Mean Diff., mean difference; n, number of patients; SD, standard deviation.
Discussion
This study introduces a novel comparison between low-energy femtosecond laser with conventional phacoemulsification addressing pupil area changes and cataract grade. Furthermore, the study is based on an intraindividual comparative assessment.
Laser-induced miosis has been associated with FLACS, with most of the literature mainly involving high-energy femtosecond laser devices.16,17
Our results add some more evidence to recently published literature18–20 pointing to an absence of laser-induced pupil constriction (pupil size immediately after laser application did not change) with the low-energy and high-frequency type of femtosecond pulse, as opposed to the high-energy and low-frequency kind, which often demands the perioperative use of supplementary anti-inflammatory drugs.21,22 The rationale for this contrast is liable to reside on the “low-energy” concept: the use of a high numerical aperture in the femtosecond laser optics, enabling the production of smaller laser spots, which, in turn, allows a reduction of the collateral damage to the neighbour tissues. Furthermore, the absence of preoperative anti-inflammatory drugs, as established in the authors’ protocol in other studies,12 did not reflect on pupil constriction, also corroborated by recent published articles.23
Cataract grade influences the energy level of ultrasound energy employed, according to the literature.15,24 The impact of higher levels of energy (for harder cataracts) may be accountable for pupillary constriction, by means of the stimulated release of inflammatory mediators. Cumulative dissipated energy (CDE) has been commonly used as a measure of such ultrasound expenditure.14,15,24
In this study, it is given further evidence that cataract grade influences ultrasound energy expenditure, as it is measurable by the CDE. It is shown that there is a negative correlation between total pupil variation and CDE with conventional phacoemulsification, associating a more negative TPV with higher CDE values. It is also consensual that the use of femtosecond laser in cataract surgery reduces the amount of ultrasound (US) employed, ergo, the CDE.25,26 In agreement with this knowledge, the present study reinforces the evidence that energy expenditure relates to cataract hardness: for softer cataracts, CDE levels are expectedly low in CP and even more with the assistance of femtosecond laser in FLACS; for harder cataracts, the ultrasound energy requisites (CDE) are significantly high in CP. The rationale will be that lower CDE levels, by means of using femtosecond laser in cataract surgery, contribute to less pupil narrowing as compared to conventional phacoemulsification. It should be stressed that the magnitude of the detected difference, although of statistical significance, is, quantitatively, of little clinical impact on surgical practice. However, these results help to demonstrate that low-energy FLACS performs similarly to conventional phacoemulsification regarding pupil status (namely pupil constriction) and may support an evidence-based decision upon choosing this technique in cataract surgery.
Regarding total pupil variation and age, no statistically significant difference was found between CP and FLACS, in the present study. However, concerning age subgroups and TPV, a statistically significant difference was shown between younger and older patients, similar for each technique. Our findings may be explained by the well-known physiological profile of the ageing pupil,27–29 characterized by a lesser pupil responsiveness and slower recovery from dilation, traduced by lower TPV values in the older subgroup of patients.
The limitations inherent to this study are the following: a suboptimal (as very large series are in demand to reinforce these findings) sample size of eyes and the assessment of prostaglandins levels. Nevertheless, the clinical evidence here collected should stimulate further studies regarding the thorough evaluation of the specific profile and role of low-energy femtosecond laser in cataract surgery.
Conclusion
The present study brings new evidence regarding the association of cataract grade with pupil status during cataract surgery. In particular, it shows a statistically significant difference in pupil behavior according to cataract grade, between CP and FLACS, favorable to the latter. Although of mild clinical relevance, this evidence does deliver a new perspective upon the real effects of the different types of femtosecond laser, thus optimizing the indications and choice of the cataract surgical technique.
Acknowledgments
João Duarte Reis, MSc, Biostatistics, Coimbra, Portugal, provided statistical support.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dick HB, Schultz T. A review of laser-assisted versus traditional phacoemulsification cataract surgery. Ophthalmol Ther. 2017;6:7–18. doi: 10.1007/s40123-017-0080-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Day AC, Burr JM, Bennett K, et al. Femtosecond laser-assisted cataract surgery compared with phacoemulsification: the FACT non-inferiority RCT. Health Technol Assess. 2021;25(6):1–68. doi: 10.3310/hta25060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grewal DS, Schultz T, Basti S, Dick HB. Femtosecond laser-assisted cataract surgery–current status and future directions. Surv Ophthalmol. 2016;61:103–131. doi: 10.1016/j.survophthal.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 4.Agarwal K, Hatch K. Femtosecond laser assisted cataract surgery: a review. Semin Ophthalmol. 2021;36(8):618–627. doi: 10.1080/08820538.2021.1890792 [DOI] [PubMed] [Google Scholar]
- 5.Diakonis VF, Yesilirmak N, Sayed-Ahmed IO, et al. Effects of femtosecond laser-assisted cataract pretreatment on pupil diameter: a comparison between three laser platforms. J Refract Surg. 2016;32:84–88. doi: 10.3928/1081597X-20151229-03 [DOI] [PubMed] [Google Scholar]
- 6.Kanclerz P, Alio JL. The benefits and drawbacks of femtosecond laser-assisted cataract surgery. Eur J Ophthalmol. 2021;31(3):1021–1030. doi: 10.1177/1120672120922448 [DOI] [PubMed] [Google Scholar]
- 7.Popiela MZ, Young-Zvandasara T, Nidamanuri P, Moore T, Leccisotti A, Kumar V. Factors influencing pupil behavior during femtosecond laser assisted cataract surgery. Cont Lens Anterior Eye. 2019;42(3):295–298. doi: 10.1016/j.clae.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 8.Wu BM, Williams GP, Tan A3, Mehta JS. A comparison of different operating systems for femtosecond lasers in cataract surgery. J Ophthalmol. 2015;2015:616478. doi: 10.1155/2015/616478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewe SY, Abell RG, Vote BJ. Femtosecond laser-assisted versus phacoemulsification for cataract extraction and intraocular lens implantation: clinical outcomes review. Curr Opin Ophthalmol. 2018;29:54–60. doi: 10.1097/ICU.0000000000000433 [DOI] [PubMed] [Google Scholar]
- 10.Lundstrom M, Dickman M, Henry Y, et al. Femtosecond laser-assisted cataract surgeries reported to the European registry of quality outcomes for cataract and refractive surgery: baseline characteristics, surgical procedure, and outcomes. J Cataract Refract Surg. 2017;43:1549–1556. doi: 10.1016/j.jcrs.2017.09.029 [DOI] [PubMed] [Google Scholar]
- 11.Mirshahi A, Ponto K. Changes in pupil area during low-energy femtosecond laser-assisted cataract surgery. J Ophthalmic Vis Res. 2019;14(3):251–256. doi: 10.18502/jovr.v14i3.4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salgado R, Torres PF, Marinho A. Pupil status with low-energy femtosecond laser-assisted cataract surgery versus conventional phacoemulsification: an intraindividual comparative study. Clin Ophthalmol. 2023;17:331–339. doi: 10.2147/OPTH.S399788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Germano C, Germano R, Cid F, Germano FA, Carricondo P, Germano J. Comparison of pupillary diameter variation between conventional phacoemulsification versus femtosecond laser-assisted cataract surgery. Arq Bras Oftalmol. 2022;85(1). doi: 10.5935/0004-2749.20210091 [DOI] [Google Scholar]
- 14.Feng L, Zhao F, Ke X, Zhao J, Shi M. Correlation between degree of lens opacity and the phacoemulsification energy parameters using different imaging methods in age-related cataract. Transl Vis Sci Technol. 2022;11(3):24. doi: 10.1167/tvst.11.3.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bui AD, Sun Z, Wang Y, et al. Factors impacting cumulative dissipated energy levels and postoperative visual acuity outcome in cataract surgery. BMC Ophthalmol. 2021;21(1):439. doi: 10.1186/s12886-021-02205-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Su F, Wang Y, Chen Y, Chen Q, Li F. Intra and post-operative complications observed with femtosecond laser-assisted cataract surgery versus conventional phacoemulsification surgery: a systematic review and meta-analysis. BMC Ophthalmol. 2019;19(1):177. doi: 10.1186/s12886-019-1190-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popovic M, Campos-Möller X, Schlenker MB, Ahmed II. Efficacy and safety of femtosecond laser-assisted cataract surgery compared with manual cataract surgery: a meta-analysis of 14 567 eyes. Ophthalmology. 2016;123(10):2113–2126. doi: 10.1016/j.ophtha.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 18.Lin H-Y, Chuang Y-J, Lin P-J. Surgical outcomes with high and low pulse energy femtosecond laser systems for cataract surgery. Sci Rep. 2021;11(1):9525. doi: 10.1038/s41598-021-89046-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirshahi A, Schneider A, Latz C, Ponto KA, Liu Y-C. Perioperative pupil size in low-energy femtosecond laser-assisted cataract surgery. PLoS One. 2021;16(5):e0251549. doi: 10.1371/journal.pone.0251549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salgado R, Torres P, Marinho A. Femtosecond laser-assisted lens surgery with low-energy pulse versus conventional phacoemulsification for presbyopia correction: an Intraindividual Study. Open Ophthalmol J. 2021;15(1):43–53. doi: 10.2174/1874364102115010043 [DOI] [Google Scholar]
- 21.Jun JH, Bang SP, Yoo YS, Joo CK. Efficacy of 0.015% intracameral epinephrine for significant miosis induced by photodisruption during femtosecond laser-assisted cataract surgery. Medicine. 2018;97(31):e11693. doi: 10.1097/MD.0000000000011693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz T, Joachim SC, Szuler M, Stellbogen M, Dick HB. NSAID pretreatment inhibits prostaglandin release in femtosecond laser-assisted cataract surgery. J Refract Surg. 2015;31:791–794. doi: 10.3928/1081597X-20151111-01 [DOI] [PubMed] [Google Scholar]
- 23.Schwarzenbacher L, Schartmüller D, Leydolt C, Menapace R. Prostaglandin release after low-energy femtosecond laser-assisted cataract surgery without anti-inflammatory drug premedication. Am J Ophthalmol. 2022;238:103–109. doi: 10.1016/j.ajo.2022.01.002 [DOI] [PubMed] [Google Scholar]
- 24.Shajari M, Rusev V, Mayer W, Diakonis V, Petermann K, Kohnen T. Impact of lens density and lens thickness on cumulative dissipated energy in femtosecond laser-assisted cataract surgery. Lasers Med Sci. 2019;34(6):1229–1234. doi: 10.1007/s10103-019-02715-6 [DOI] [PubMed] [Google Scholar]
- 25.Yesilirmak N, Diakonis VF, Batlle JF, et al. Comparison of phacoemulsification parameters between manual and femtosecond laser-assisted cataract surgery. Can J Ophthalmol. 2018;53(5):542–547. doi: 10.1016/j.jcjo.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 26.Saeedi OJ, Chang LY, Ong SR, et al. Comparison of cumulative dispersed energy (CDE) in femtosecond laser-assisted cataract surgery (FLACS) and conventional phacoemulsification. Int Ophthalmol. 2019;39(8):1761–1766. doi: 10.1007/s10792-018-0996-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baldascino A, Carlà MM, Giannuzzi F, et al. Femtosecond laser-assisted cataract surgery: analysis of surgical phases and comparison with standard phacoemulsification in uncomplicated cataracts. Vision. 2022;6(4):72. doi: 10.3390/vision6040072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fotiou DF, Brozou CG, Tsiptsios DJ, et al. Effect of age on pupillary light reflex: evaluation of pupil mobility for clinical practice and research. Electromyogr Clin Neurophysiol. 2007;47(1):11–22. [PubMed] [Google Scholar]
- 29.Lee YS, Kim HJ, Lim DK, Kim MH, Lee KJ. Age-specific influences of refractive error and illuminance on pupil diameter. Medicine. 2022;101(27):e29859. doi: 10.1097/MD.0000000000029859 [DOI] [PMC free article] [PubMed] [Google Scholar]