Abstract
Aim:
This study aimed at assessing the efficacy of targeted interventions addressing common food sensitivities and lifestyle factors that commonly contribute to the presentation of gastrointestinal problems identified as Irritable bowel syndrome (IBS).
Background:
IBS has served to cover the expression of multifactorial disorders with variable aetiology and pathophysiology. Food antigens implicated in the modern lifestyle, acting as strong epigenetic factors is strongly implicated in pathophysiology of conditions under IBS. Identifying and addressing food sensitivities in patients presenting with IBS like symptoms are currently underemphasised in clinical guidelines yet have the potential to provide major benefits for patients.
Methods:
Information was collected from the medical records of patients that were referred to the Gastroenterology Unit of Palmerston North DHB with unexplained gastrointestinal (GI) symptoms with or without other GI comorbidities between September 2018 and November 2021.
Results:
The main management option offered to the 121 patients included in this study, was lifestyle adjustment and/or a trial of 6 weeks, eliminating gluten and lactose from the diet. The most prevalent symptoms were abdominal pain 96/121 (79%), diarrhoea 83/121 (69%), followed by bloating and constipation. Seventy-eight patients had the outcomes of their improvement available. A total of 42 out of 78 patients (54%) were treated exclusively with gluten and lactose-free diet, in this group of patients 86% (36/42) reported a significant improvement in their symptoms with a score in the range of 40-100%.
Conclusion:
Our study illustrates the importance of focusing on triggering factors when assessing patients with IBS. We suggest that careful identifying and eliminating the triggering food antigens as monotherapy or in addition to the lifestyle adjustment where appropriate should be the main objective in symptomatic patients fulfilling the IBS diagnostic criteria. These combinations and holistic approach in treating IBS’ patients’ symptoms are less expensive, non-toxic, and highly effective in achieving optimal outcomes and improving these patient’s quality of life.
Key Words: IBS, Gluten, Non-coeliac gluten sensitivity, Lactose intolerance
Introduction
Before modern diagnostic technology, gastrointestinal symptoms without obvious etiology were labelled under the term Irritable bowel syndrome (IBS) (1). IBS was described as a tension between nervous system and abdomen. Patients invariably have interrelated problems that are multifactorial and complex. The term IBS has served to cover the expression of several recently identified disorders with variable aetiology and pathophysiology (2-4). Therefore, consensus-based clinical diagnostic tools have not been effective in bringing a unified clarity in disease behavior (5).
The multifactorial nature of IBS includes stressful life events and implicate interaction between biological, including the gut microbiota (6), psychological and social lifestyle factors. From early days antispasmodics, low-residue diet, sedatives, laxatives and antacids were recommended (1, 7). The diversity of food antigens implicated in the modern lifestyle act as strong epigenetic factors bring in light the pathophysiology of conditions identified under the term IBS. Despite strong implications of food antigens like gluten (8) and beta-casein (lactose intolerance (9)) in this condition, symptomatic treatment with expensive medications is still the major therapeutic intervention recommended in most of clinical guidelines (10). Prescribing analysis and cost tabulation (PACT) data in 2012-2013 indicated that £44 977 959 and £25 582 752, respectively, were spent on selected laxatives and antispasmodics commonly used to treat IBS in primary care in UK (11). This is why an etiology based intervention algorithm (12) has been proposed to treat symptoms by identifying triggering factors (13) to reduce prescribing of these expensive medications and related adverse drug reactions (14). There is a strong association between symptoms of IBS and other conditions’ symptoms like coeliac disease (15) or anxiety and depression (16). These patients are vulnerable and those with severe symptoms are willing to take significant risks for medication side effect in desperation (17). This study reports the outcome of individualised treatment and care intervention based on true presentation of each patient’s lifestyle and triggering factors that commonly contribute to the presentation of gastrointestinal symptoms identified as IBS.
Methods
We performed a retrospective study on 121 patients presenting with symptoms consistent with IBS at the Palmerston North Hospital, New Zealand, from September 2018 through November 2021. In this study 73% were female and 27% male; age range 18-88). Ethnicity wise, 74/121 were NZ European (61%), 9/121 (7%) were NZ Māori, 4/121 (3%) Asian and 29/121 (24%) were from another European ethnicity. The outcome data were available for 78/121. Patients were thoroughly assessed, actively looking for patient related risk/triggering factors instead of stereotyping them with IBS marker. Individual based socio lifestyle habit were studied for each individual including BMI in addition to taking in consideration possible sensitivity to potential food antigens like gluten, lactose and those nutrients included in FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols).
If lifestyle modification like adequate mastication, reduced portion size, avoiding triggering factors like sedentarism, consumption of food high in sugar and fat, caffeine or high alcohol intake was not indicated or effective, an elimination diet was recommended. The first elimination diet consisted of a 6-week gluten and lactose free diet guided by a dietician. Individuals who did not respond adequately to elimination of lactose and gluten, were offered a further elimination diet with FODMAP. The primary endpoint was mean change in presenting Symptom Severity Score; a 30% reduction was considered to indicate a clinical response (18). Secondary endpoints were changes in anxiety and depression score, fatigue impact score, and headache. In this study we evaluated the number of medications that were prescribed and the number of follow-ups appointments.
Statistical analysis
The data are summarised by tabulation and upset plots. Tables shown the proportion of patients at various symptoms and the situation of intervention (Table 1-4). Upset plot is used for visualizing the overlap and intersections between corresponding symptoms, extraintestinal symptoms, and type of interventions separately (Figures 1-3). Plots identify the common patients or relationships among multiple categories, and to explore the distribution and frequency of these patients across different categories. All analysis is done by R 4.21 (R Core Team, 2022) and its package ‘ComplexUpset’ (M. Krassowski, 2020) (19, 20).
Table 1.
Presenting symptoms and improvement rate
| Symptoms | Patients | Improvement |
|---|---|---|
| Abdominal pain | 67/78 | 55(82%) |
| Diarrhoea | 57/78 | 48(84%) |
| Bloating | 39/78 | 31(79%) |
| Constipation | 21/78 | 19(90%) |
| Weight-loss | 12/78 | 11(92%) |
| Reflux | 12/78 | 10(83%) |
| Nausea | 11/78 | 8(73%) |
| Vomiting | 8/78 | 8(100%) |
Table 4.
BMI and symptoms improvement following intervention
| BMI | Improvement | No improvement | NA | Total |
| Underweight (<18.5) | 3 | 0 | 1 | 4 |
| Healthy (18.5-24.9) | 12 | 4 | 15 | 31 |
| Overweight (25-29.9) | 20 | 2 | 5 | 27 |
| Obese (30 and >30) | 14 | 3 | 10 | 27 |
| BMI not available | 17 | 4 | 11 | 32 |
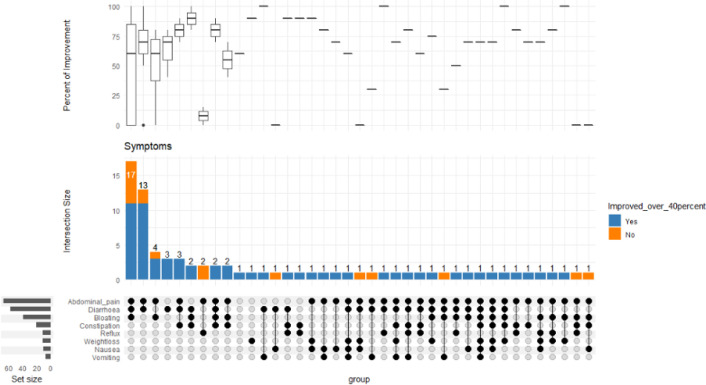
Figure 1.
Bottom left represents the total no. of patients with the corresponding symptom. The middle bar chart represents no. of patients with the corresponding intersection of symptoms. The most frequent symptoms in this group of patients are abdominal pain, diarrhoea and constipation. Box plot represents the distribution of the percentage of improvement across the various symptom intersections
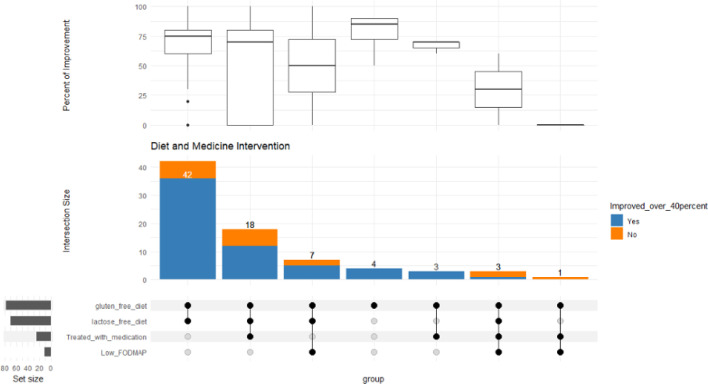
Figure 3.
Bottom left represents the total no. of patients offered dietary interventions (gluten-free diet, lactose-free diet and low FODMAP diet), also those treated with medication. The middle bar chart represents no. of patients with the corresponding intersection of offered treatment. The most commonly offered treatment was a gluten and lactose-free diet, followed by gluten and lactose-free diet with medication. Box plot represents the distribution of the percentage of improvement across the various treatment intersections
Results
Lifestyle adjustment was effective in combination with elimination diet as highlighted in Table 3. A trial of 6 weeks, strict eliminating gluten and lactose from the diet and/or lifestyle adjustment were the main therapeutic recommendations. To estimate the symptoms prevalence in larger number of patients the calculation was based on 121 participants, but the intervention outcome was available in 78 patients as illustrated in Table 1.
Table 3.
Individual based intervention directed to potential etiology
| Patient Improvement | Improved | NA | |
|---|---|---|---|
| Interventions | Yes | No | NA |
| Medications | 35 | 85 | 1 |
| Mindfulness | 5 | 114 | 2 |
| Decreased portion sizes | 7 | 112 | 2 |
| Weight loss | 12 | 107 | 2 |
| Exercise | 12 | 107 | 2 |
| Other Lifestyle Interventions | 30 | 89 | 2 |
| Gluten free Diet | 115 | 5 | 1 |
| Lactose free Diet | 102 | 17 | 2 |
| Low FODMAP diet | 12 | 107 | 2 |
| Other sensitivities | 17 | 102 | 2 |
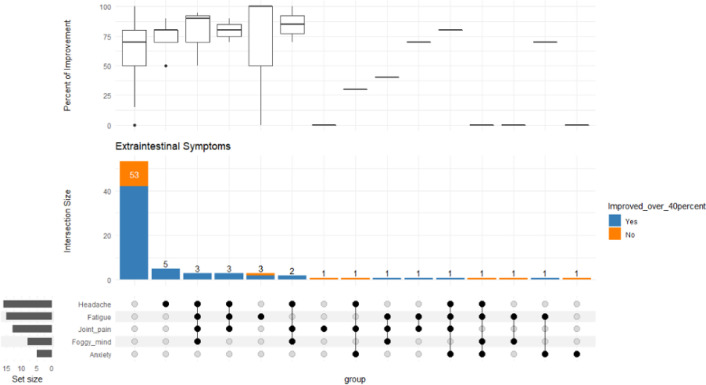
The most prevalent symptoms were abdominal pain 96/121 (79%), diarrhoea 83/121 (69%), followed by bloating and constipation. Seventy-eight patients had the outcomes of their improvement available. A total of 42 out of 78 patients (54%) were treated exclusively with gluten and lactose-free diet, in this group of patients 86% (36/42) reported a significant improvement in their symptoms with a score in the range of 40-100% (see Figure 1). The extraintestinal symptoms improvement were in the range of 60-94% (see Table 2 and Figure 2). Anxiety scored as the least improved symptom in the range of 60%.
Table 2.
Presenting extraintestinal symptoms and improvement rate
| Extraintestinal symptoms | Patients | Improvement |
|---|---|---|
| Fatigue | 15/78 | 12(80%) |
| Headache | 16/78 | 15(94%) |
| Joint pain | 13/78 | 12(92%) |
| Foggy mind | 8/78 | 6(75%) |
| Anxiety | 5/78 | 3(60%) |
Figure 2.
Bottom left represents the total no. of patients with each extraintestinal symptom. The middle bar chart represents no. of patients with the corresponding intersection of symptoms. The majority of patients had no extraintestinal symptoms, the most common intersection of extraintestinal symptoms, however, had headaches only, followed by those with headache, fatigue, joint pain and a foggy mind. Box plot represents the distribution of the percentage of improvement across the various symptom intersections
No medications was prescribed in over 70% of this study group. The main prescribed medication in 28% of patients were fibre-based laxative (Table 3). Other interventions included low FODMAP diet as the second line nutrition therapy, recommendation for mindfulness, weight reduction and physical exercises as summarised in Table 3 and Figure 3. BMI data was available for 89/121 patients of this patient group. From this group 31/89 (35%) had normal BMI, 27/89 (30%) were overweight and 30% had a BMI of >30 (Table 4). Symptom improvement in patients with a BMI in overweight and obese ranges were 20/22 (90%) and 14/17 (82%) respectively. None of the 3 patients with a BMI <18 showed significant improvement.
Discussion
Conditions under the label of IBS are often multifactorial and labelling these patients with a diagnosis of IBS might be misleading health professional and patients to achieve the right diagnosis. IBS treatment strategy is mainly focused on symptomatic treatment rather than treatment of potential implicated factors in the pathogenesis of disease. In this study, the term IBS was avoided, and patients were assessed based on their presentation and individual characteristics implicated in their conditions. We identified that the gastrointestinal symptoms were often associated with other factors like individual lifestyles including sedentarism, high BMI, reduced physical activities, poor mastication habit, food sensitivities, lack of sleep, anxiety and depression. Identifying above mentioned factors without using the term IBS has created a more effective approach to the practical therapeutic insight.
It was clear for instance that masking the food sensitivities under the label of IBS could prevent reaching the correct diagnosis of conditions like lactose intolerance (LI) which is highly prevalent in European, American, Asian, and African populations with a prevalence of 15%, 50%, 70%, and 100%, respectively (21-23). What a patient with LI needs is a lactose-free diet not an anti-diarrhoeal, spasmolytic/antiemetic or laxative. Similar mislabeling has been reported in patients with non-coeliac gluten sensitivity (NCGS) (18, 24-26).
Using an algorithm (12) to identify the potential risk factors for patients in this study was associated with highly effective individualised treatment recommendations with a positive outcome scoring between 40-100% improvement. This approach not only reduced prescribing expensive medications with well-known adverse reactions, but it was also associated with undertaking less expensive unnecessary investigations for patients fulfilling the Rome IV criteria (27) and significantly reduced number of follow up appointments. In addition to a low side effect profile with reduced number of follow-ups and cost saving, this modality of assessment and care gave ownership to patients to control their condition by undertaking healthy steps for their short and long term improvement leading to higher patient satisfaction. Many patients were obese or overweight with good response to lifestyle adjustment that included increased physical activity and mild to moderate weight reduction (see Table 4).
In contrast to the recent British Society of Gastroenterology (BSG) guideline where gluten elimination is not recommended for patients presenting with IBS (28), in this study we identified that a large number of patients were gluten sensitive. It is important to note that some patients presented to clinic with persistent symptoms despite being on a gluten and lactose free diet. A dietary evaluation often demonstrated that these patients were on reduced gluten or lactose intake and not on fully restricted gluten or lactose elimination as they were unaware of potential contaminations. Adequate guidance on strict gluten/lactose restriction was an important intervention leading to improving of a good number of these patients before transition to implementing other restrictions like low FODMAP.
The most likely explanation for these patients’ improvement following a full gluten/lactose restriction is the fact that even in NCGS, the threshold of gluten tolerance is variable, and many patients may react to traces of gluten keeping them symptomatic.
Since most of these patients respond to a strict elimination diet, a strict gluten and lactose exclusion diet under the guidance of a well-trained dietitian should be mandatory before applying further restriction and elimination diet. We have shown in this study, patients presenting with symptoms compatible with IBS may have different etiology related to lifestyle and food sensitivity. Hence, the focus of treatment must be broadened to encompass the associated effects of stress, behaviour, personality, and food sensitivities (12, 29). Therefore, using IBS as a diagnosis and applying symptomatic treatment might not only be misleading and ineffective but is also expensive and puts patients at risk of the side effects of these medications and long-term unnecessary invasive diagnostic interventions. A solid reassuring physician-patient relationship is the cornerstone for any effective strategy in identifying appropriate individualized intervention (12) for each patient (12, 30, 31).
Conflict of interests
There are no conflicts of interest to disclose for any authors.
Acknowledgments
We would like to thank Dr. Jack Yee, Gastroenterology Registrar for his contribution by supporting Taliah Sua in data collection.
References
- 1.Whitcomb Jr FF, Cain JC. Irritable bowel. Postgrad Med. 1963;33:233–236. doi: 10.1080/00325481.1963.11692798. [DOI] [PubMed] [Google Scholar]
- 2.Rostami K, Ensari A, Marsh MN, Srivastava A, Villanacci V, Carroccio A, et al. Gluten induces subtle histological changes in duodenal mucosa of patients with non-coeliac gluten sensitivity: a multicentre study. Nutrients. 2022;14:2487. doi: 10.3390/nu14122487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuppan D. Wheat Syndromes: How Wheat, Gluten and ATI Cause Inflammation, IBS and Autoimmune Diseases. 1st ed. Springer ebool; pp. 57–91. [Google Scholar]
- 4.Lomer MC, Parkes G, Sanderson J. lactose intolerance in clinical practice–myths and realities. Aliment Pharmacol Ther. 2008;27:93–103. doi: 10.1111/j.1365-2036.2007.03557.x. [DOI] [PubMed] [Google Scholar]
- 5.Blomhoff S, Diseth TH, Jacobsen MB, Vatn M. [Irritable bowel syndrome--a multifactorial disease in children and adults] Tidsskrift for den Norske laegeforening: tidsskrift for praktisk medicin, ny raekke. 2002;122:1213–1217. [PubMed] [Google Scholar]
- 6.Dupont H. Evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther. 2014;39:1033–1042. doi: 10.1111/apt.12728. [DOI] [PubMed] [Google Scholar]
- 7.Watson B. What is IBS? 1964. [Google Scholar]
- 8.Shahbazkhani B, Sadeghi A, Malekzadeh R, Khatavi F, Etemadi M, Kalantri E, et al. Non-celiac gluten sensitivity has narrowed the spectrum of irritable bowel syndrome: a double-blind randomized placebo-controlled trial. Nutrients. 2015;7:4542–4554. doi: 10.3390/nu7064542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong L, Wang Y, Gong X, Chen M. Prevalence of lactose intolerance in patients with diarrhea-predominant irritable bowel syndrome: data from a tertiary center in southern China. J Health Popul Nutr. 2017;36:1–5. doi: 10.1186/s41043-017-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasant DH, Paine PA, Black CJ, Houghton LA, Everitt HA, Corsetti M, et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut. 2021;70:1214–1240. doi: 10.1136/gutjnl-2021-324598. [DOI] [PubMed] [Google Scholar]
- 11.Soubieres A, Wilson P, Poullis A, Wilkins J, Rance M. Burden of irritable bowel syndrome in an increasingly cost-aware National Health Service. Frontline Gastroenterol. 2015;6:246–251. doi: 10.1136/flgastro-2014-100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rostami K, Bold J, Ismail Ali J, Parr A, Dieterich W, Zopf Y, et al. An algorithm for differentiating food antigen-related gastrointestinal symptoms. Gastroenterol Hepatol Bed Bench. 2021;14:8–16. [PMC free article] [PubMed] [Google Scholar]
- 13.Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;2:653–654. doi: 10.1136/bmj.2.6138.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lembo A. Irritable bowel syndrome medications side effects survey. J Clin Gastroenterol. 2004;38:776–781. doi: 10.1097/01.mcg.0000139029.00451.c7. [DOI] [PubMed] [Google Scholar]
- 15.Sanders D. Irritable bowel syndrome and coeliac disease. Lancet. 2002;359:1436–1437. doi: 10.1016/S0140-6736(02)08378-2. [DOI] [PubMed] [Google Scholar]
- 16.Karling P, Danielsson Å, Adolfsson R, Norrback Kf. No difference in symptoms of irritable bowel syndrome between healthy subjects and patients with recurrent depression in remission. Neurogastroenterol Motil. 2007;19:896–904. doi: 10.1111/j.1365-2982.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 17.Lacy BE, Everhart KK, Weiser KT, DeLee R, Strobel S, Siegel C, et al. IBS patients' willingness to take risks with medications. Am J Gastroenterol. 2012;107:804–809. doi: 10.1038/ajg.2011.485. [DOI] [PubMed] [Google Scholar]
- 18.Catassi C, Elli L, Bonaz B, Bouma G, Carroccio A, Castillejo G, et al. Diagnosis of non-celiac gluten sensitivity (NCGS): the Salerno experts’ criteria. Nutrients. 2015;7:4966–4977. doi: 10.3390/nu7064966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krassowski M. ComplexUpset: create complex UpSet plots using ggplot2 components. R package version 05 2020;18. Available from: https://cran.r-project.org/web/packages/ComplexUpset/ComplexUpset.pdf.
- 20.Team RDC. A language and environment for statistical computing. 2009. Available from: http://www.R-project.Org.
- 21.Storhaug CL, Fosse SK, Fadnes LT. Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:738–746. doi: 10.1016/S2468-1253(17)30154-1. [DOI] [PubMed] [Google Scholar]
- 22.Rosado JL. Intolerancia a la lactosa. Gac Med Mex. 2016;152:67–73. [PubMed] [Google Scholar]
- 23.Montes P, Monge E. Lactose malabsorption and IBS. Scand J Gastroenterol. 2004;39:1033–1033. doi: 10.1080/00365520410009627. [DOI] [PubMed] [Google Scholar]
- 24.Fasano A, Sapone A, Zevallos V, Schuppan D. Nonceliac gluten sensitivity. Gastroenterology. 2015;148:1195–1204. doi: 10.1053/j.gastro.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 25.Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:1–12. doi: 10.1186/1741-7015-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmes G. Non coeliac gluten sensitivity. Gastroenterol Hepatol Bed Bench. 2013;6:11511–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Palsson OS, Whitehead WE, Van Tilburg MA, Chang L, Chey W, Crowell MD, et al. Rome IV diagnostic questionnaires and tables for investigators and clinicians. Gastroenterology. 2016:S0016–5085 . doi: 10.1053/j.gastro.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Vasant DH, Paine PA, Black CJ, Houghton LA, Everitt HA, Corsetti M, et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut. 2021;70:1214–1240. doi: 10.1136/gutjnl-2021-324598. [DOI] [PubMed] [Google Scholar]
- 29.Drossman DA. Irritable bowel syndrome: a multifactorial disorder. Hosp Pract. 1988;23:119–133. doi: 10.1080/21548331.1988.11703538. [DOI] [PubMed] [Google Scholar]
- 30.Occhipinti K, Smith JW. Irritable bowel syndrome: a review and update. Clin Colon Rectal Surg. 2012;25:046–052. doi: 10.1055/s-0032-1301759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adriani A, Ribaldone DG, Astegiano M, Durazzo M, Saracco GM, Pellicano R. Irritable bowel syndrome: the clinical approach. Panminerva Med. 2018;60:213–222. doi: 10.23736/S0031-0808.18.03541-3. [DOI] [PubMed] [Google Scholar]