Figure 4.
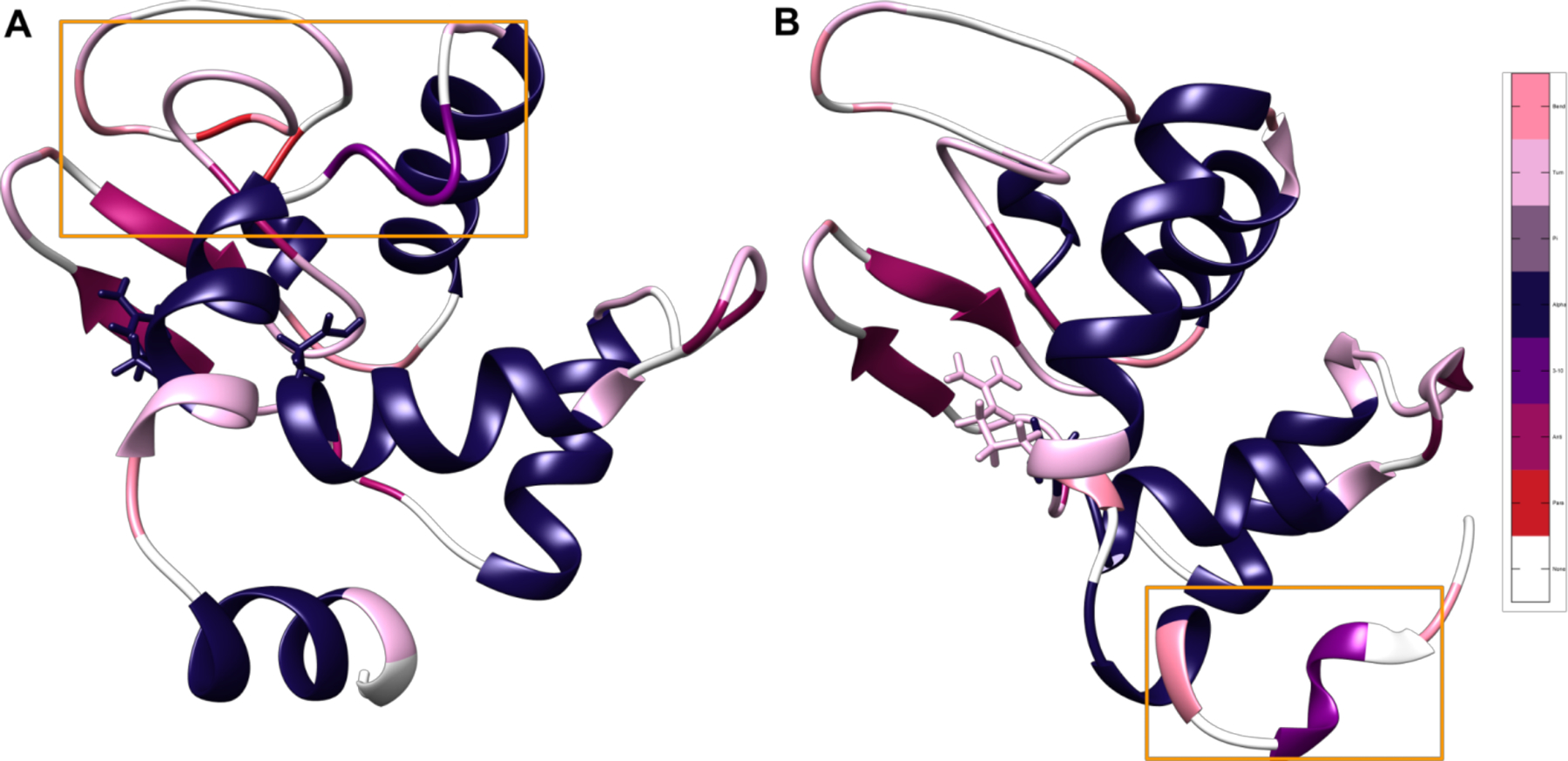
Most frequently occurring structures in the cluster corresponding to the distance between the broken disulfide bond between residues 6 and 127. Representative structures for the two protonation states, colored by the most common secondary structure after the 5 μs mark, with salmon representing bend character, pink representing turn character, gray representing pi helix character, navy blue representing α helix character, purple representing 3–10 helix character, mauve representing antiparallel β sheet, red representing parallel β sheet, and white representing unstructured loops. Glu35 and its corresponding Arg114 hydrogen bonding partner are displayed in licorice. Areas that are particularly different between states are highlighted with orange boxes. The difference in location of unfolding indicates the presence of two divergent unfolding pathways based on protonation state and subsequent hydrogen bonding. (A) State 1 with all disulfide bonds broken and the Glu35 hydrogen on OE1. (B) State 2 with all disulfide bonds broken and the Glu35 hydrogen on OE2.
