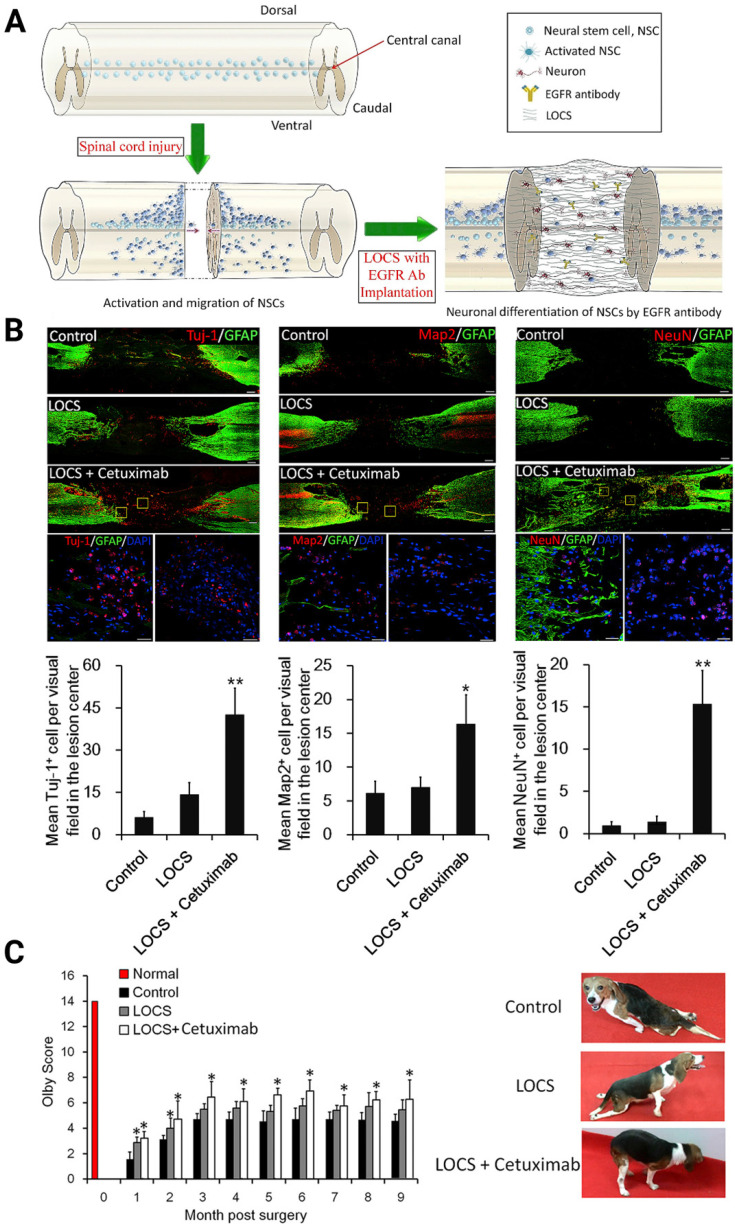
Figure 6.
(A) Schematic of injury-induced activation and migration of NSPCs and cetuximab-promoted neuronal differentiation of NSPCs. Endogenous NSPCs remain quiescent in the intact spinal cord, but severe SCI can activate NSPCs and induce their migration into the lesion site. Blocking EGF signaling can promote the injury-activated NSPCs to differentiate into neurons. These newborn neurons can then build neuronal relays to bridge injury gaps and lead to functional recovery after SCI. (B) Endogenous neurogenesis and CSPG deposition in lesion sites of dogs with long-term T8 removal injury. Upper panels: Tuj-1-positive newborn neurons, MAP2+ mature neurons, and NeuN+ cells in the lesion sites of dogs in the control, scaffolds implantation and cetuximab-modified scaffolds implantation (LOCS+Cetuximab) groups at 9 months after injury. Bottom panels: enlargements of areas indicated in AeC (yellow boxes), and quantification of newborn neurons, mature neurons, and neuronal nuclei in lesion centers of dogs among each group. (C) Locomotion recovery, assessed with Olby scores, at each time point examined. Adapted with permission from 42, Copyright 2017 Elsevier.