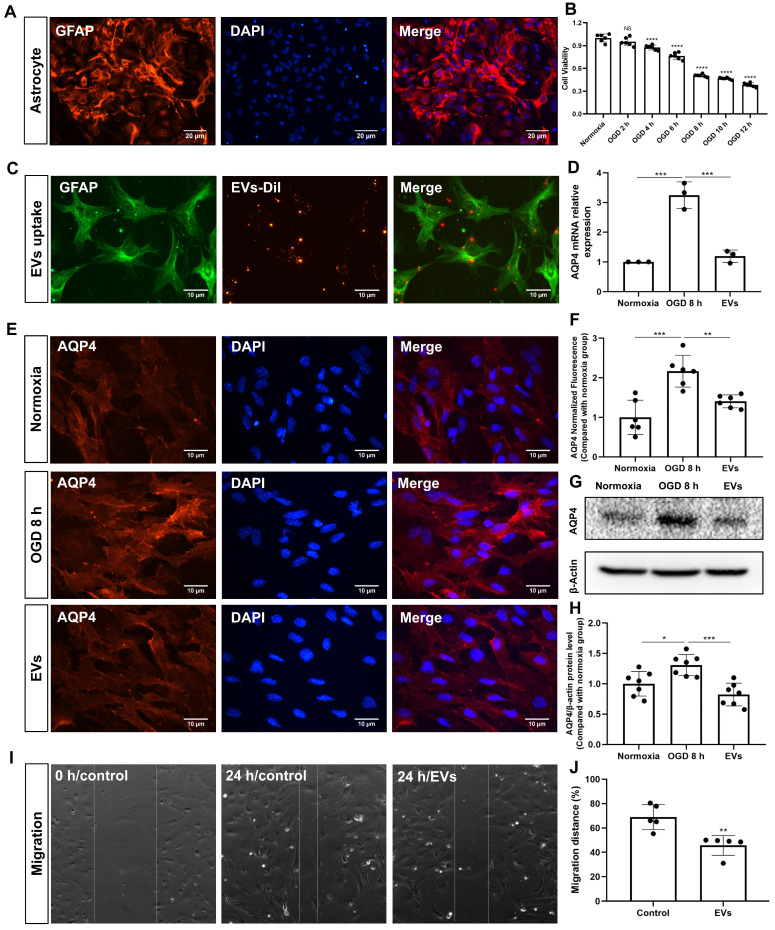
Figure 5.
EV treatment reduces the clustering of AQP4 in the astrocyte plasma membrane exposed to hypoxia. (A) Identification of primary cortical astrocytes. Cell cultures were immunoassayed for GFAP (red) and counterstained with DAPI (blue). (B) MTT (Thiazolyl Blue Tetrazolium Bromide) was used to assess the astrocyte viability exposed to 2, 4, 6, 8 10, and 12 h of OGD followed by 24-h reoxygenation. Cells incubated under standard cell culture conditions ('Normoxia') were defined as 100 % cell survival (n = 6). (C) EVs labeled with DiI (red) were taken up into the cytoplasm of GFAP+ (green) astrocytes. (D) RT-qPCR assay of the impact of EV treatment of AQP4 gene expression in primary astrocytes exposed to 8 h of OGD followed by 24 h of reoxygenation (n = 3). (E-F) Immunocytochemistry with the antibody specific for AQP4 confirmed AQP4 protein clustering in the plasma membrane under different treatment conditions (n = 6). (G-H) Western blot analysis showing the AQP4 protein in primary astrocytes after OGD in untreated cells or cells treated with EVs (n = 7). (I-J) EVs decreased the capability of migration of astrocytes in the scratch wound model (24 h, n = 5). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; NS, not statistically significant; OGD, oxygen-glucose deprivation; EVs, extracellular vesicles; AQP4, aquaporin 4; RT-qPCR, quantitative real-time PCR analysis.