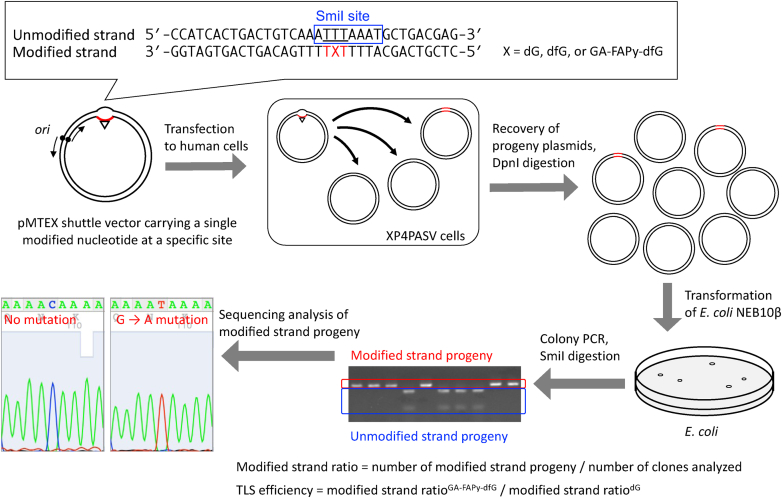
Figure 5.
Schematic diagram of the intracellular TLS assay using a shuttle vector. dG, dfG, and GA-FAPy-dfG were introduced at a specific position (denoted as X) on one side of the shuttle vector (modified strand), whereas the other strand (unmodified strand) had a three-base mismatch (underlined) for the GA-FAPy-dfG and SmiI recognition sequence (blue box). The vector was transfected into XP4PASV cells and allowed to replicate for 48 h. Progeny plasmids were then recovered and digested with DpnI to remove nonreplicated original DNA. Escherichia coli was transformed with progeny plasmids, and resultant clones were subjected to PCR to amplify the lesion site. The PCR products were treated with SmiI to digest unmodified strand progeny. Replication efficiency was calculated from the ratio of the modified strand progeny in the recovered plasmids and is shown relative to dG. Mutation spectra were analyzed using Sanger sequencing of the PCR products of the modified strand progeny. GA-FAPy-dfG, N6-(2-deoxy-2-fluoro-d-arabinofuranosyl)-2,6-diamino-3,4-dihydro-4-oxo-5-[N-(2-carbamoyl-2-hydroxyethyl)formamido]pyrimidine; TLS, translesion DNA synthesis.