Abstract
Background
Limited research has been conducted on the association between preconception exposure to ambient particulate matter (PM) and hypothyroidism. This study aimed to investigate the relationship between preconception PM exposure and hypothyroidism.
Methods
A retrospective case–control study at China-Japan Friendship Hospital was performed. Fine particulate matter (PM2.5) and inhalable particulate matter (PM10) were obtained from the China High Air Pollution Dataset. Buffer analysis methods were used to calculate the exposure of pregnant women to PM in a circular area of 250, 500, and 750 m in diameter at preconception and in early pregnancy. Logistic regression models were used to assess the relationship between PM and hypothyroidism. Odd ratios (ORs) and 95% confidence intervals (CIs) were used to evaluate the effect of PM on the risk of hypothyroidism.
Results
A total of 3,180 participants were studied, and they comprised 795 hypothyroid patients and 2,385 matched controls. The mean age was 31.01 years (standard deviation: 3.66) in the control group and 31.16 years (standard deviation: 3.71) in the case group. Logistic regression analysis showed that exposure to PM2.5 and PM10 in the 60-day period before the last menstrual period month (LMPM), 30-day period before the LMPM, and LMP, across all distance buffers, was associated with an increased risk of hypothyroidism (all P < 0.05). The most pronounced effect was observed during the LMPM, with PM2.5 (OR: 1.137, 95% CI: 1.096–1.180) and PM10 (OR: 1.098, 95% CI: 1.067–1.130) in the 250-m buffer. Subgroup analysis in the Changping District yielded consistent results with the main analysis.
Conclusion
Our study shows that preconception PM2.5 and PM10 exposure increases the risk of hypothyroidism during pregnancy.
Keywords: Hypothyroidism, Ambient particulate matter, Risk factor, Pregnancy, Air pollution, Preconception
Introduction
Hypothyroidism is a disease characterized by a reduced systemic metabolism owing to a reduction in the synthesis and secretion of thyroid hormones or a decline in the effects of thyroid hormones (Chaker et al. 2022). Hypothyroidism is an important public health problem, and studies have shown that the prevalence of hypothyroidism during pregnancy is approximately 2% in areas with adequate iodine. (Korevaar et al. 2017; Medici et al. 2015). Hypothyroidism during pregnancy has long-term or short-term adverse effects on maternal and fetal outcomes. Hypothyroidism in pregnancy is an important risk factor for adverse pregnancy outcomes, such as preterm birth (Korevaar et al. 2019), miscarriage (Zhang et al. 2017), and pediatric endocrine morbidity in the offspring (Eshkoli et al. 2019), and is a major cause of critical illnesses, such as preeclampsia in pregnancy (Su et al. 2021). Although hypothyroidism is not a direct cause of maternal death, it is still a risk factor for other diseases. Therefore, health education and prevention for pregnant women may reduce the risk of developing hypothyroidism and related complications.
In recent years, the association between diseases and air pollution has gained attention (Bai et al. 2020; Dehghani et al. 2020). The main air pollutants involved are ambient particulate matter (PM), ozone (O3), nitrogen dioxide (NO2), carbon monoxide (CO), and other trace organic substances and volatile organic compounds, among which PM has the greatest effect on human health (Dehghani et al. 2020; Orellano et al. 2020). Pregnant women represent a unique and vulnerable population when environmental stressors are present. During pregnancy, women undergo various physiological and hormonal changes, which can affect their susceptibility to the effects of environmental stressors (Soma-Pillay et al. 2016; Wang et al. 2021). Maternal exposure to PM can potentially affect the health and well-being of the mother and the developing fetus. Exposure to PM during pregnancy is associated with adverse pregnancy outcomes, such as gestational diabetes (Zhang et al. 2020), fetal growth restriction (Zhou et al. 2022b), and hypertensive disorders in pregnancy (Cao et al. 2021). Investigating the association between PM and thyroid function specifically in pregnant women is important because thyroid hormones play a crucial role in fetal brain development and overall maternal health (Huget‐Penner and Feig 2020). However, few studies have been conducted on air pollutants and hypothyroidism during pregnancy.
The pathogenesis of hypothyroidism in pregnancy is unclear, and may involve genetic factors, lifestyle, dietary, and environmental factors (Dehghani et al. 2022; Maraka et al. 2016). In recent years, there has been increasing concern regarding the potential adverse effects of PM exposure during pregnancy on thyroid function in pregnant women. A study of four European cohorts and one United States cohort showed that fine particulate matter (PM2.5) exposure during the first trimester of pregnancy was associated with mild thyroid dysfunction throughout pregnancy (Ghassabian et al. 2019). Studies have shown that maternal free thyroxine (FT4) concentrations are inversely associated with maternal exposure to PM2.5 (Wang et al. 2019), but not associated with inhalable particulate matter (PM10) (Zhang et al. 2022). Research suggests that PM2.5 may interfere with thyroid function in pregnant women during the first trimester (Zhao et al. 2019).
These studies suggest an association between PM2.5 and PM10 exposure and thyroid function in pregnant women, However, to the best of our knowledge, no studies have investigated the association between preconception exposure to ambient PM and hypothyroidism during pregnancy. Moreover, evaluations of PM in previous studies were based on city- or county-level atmospheric stations, which may not have accurately estimated the exposure of pregnant women to PM throughout pregnancy. Therefore, the association between preconception exposure to PM and the risk of hypothyroidism during pregnancy and whether there any temporal variations in this association during different time windows need to be determined. To address these issues, this study aimed to (1) investigate the relationship between preconception and early pregnancy PM exposure and the risk of hypothyroidism during pregnancy using a high-precision 1 × 1-km gridded China High Air Pollutant (CHAP) dataset (Wei et al. 2021a); and (2) to examine potential temporal variations in the association between preconception PM exposure and hypothyroidism during different time windows, comprising the 30-day period before the last menstrual period month (LMPM), the 60-day period before the LMPM, and the 90-day period before the LMPM. The findings of our study will contribute to the existing knowledge on the potential health risks associated with PM exposure during pregnancy, providing important insight into the prevention and management of maternal health risks related to PM exposure.
Methods
Participants and study design
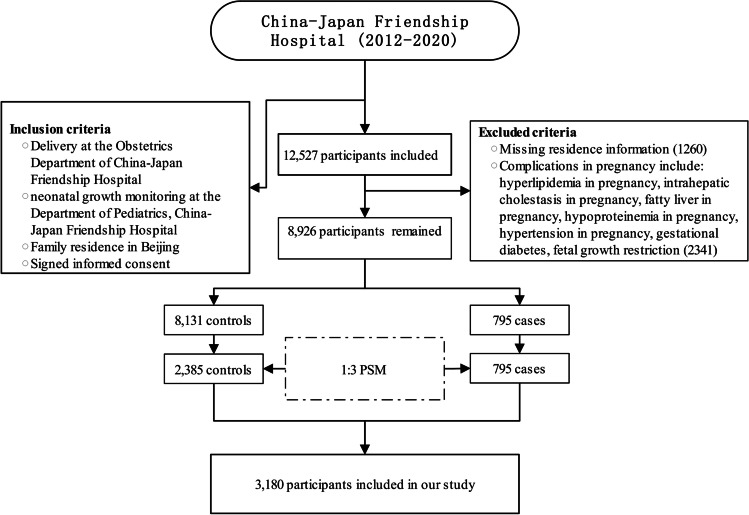
This was a case–control study conducted at China-Japan Friendship Hospital. The study population consisted of pregnant women who gave birth at this hospital between 2012 and 2020. The inclusion criteria for the study subjects were as follows: 1) delivery at the Obstetrics Department of China-Japan Friendship Hospital; 2) neonatal growth monitoring was performed at the Pediatrics Department of China-Japan Friendship Hospital after birth, 3) residence in Beijing; and 4) signed informed consent. The exclusion criteria for the study population were as follows: 1) missing residence information; and 2) the women had complications of pregnancy, such as hyperlipidemia, intrahepatic cholestasis, fatty liver, hypoproteinemia, hypertension, diabetes mellitus, and fetal growth restriction. The controls in this study were matched by propensity score matching at a ratio of 1:3 according to age, ethnicity, gravidity, and parity. The workflow of the study is shown in Fig. 1. The study protocol was approved by the Ethical Review Board of China-Japan Friendship Hospital (No: 2022-KY-006–1).
Fig. 1.
Flowchart of this study. PSM: propensity score matching
Assessment of hypothyroidism
Hypothyroidism was diagnosed on the basis of assessment of thyroid-stimulating hormone (TSH) and FT4 concentrations. The diagnostic criteria of hypothyroidism were TSH concentrations > 4 mU/L and FT4 concentrations below the lower limit of the reference range (0.93–1.70 ng/dL) (Ross 2013). Before being enrolled in the study, all participants underwent a minimum of one thyroid function test, which was conducted before the 12th week of pregnancy. Subsequently, participants who were diagnosed with hypothyroidism were classified and assigned codes in accordance with the tenth revision of the International Classification of Diseases (ICD-10) (DiSantostefano 2009).
Assessment of air pollution
The 1 × 1-km gridded CHAP monthly dataset (available at https://doi.org/10.5281/zenodo.3753614) was used to measure short-term pollutant exposure. In brief, the CHAP dataset is a long-term, full-coverage, high-resolution, high-quality, and ground-gridded source of air pollutant data. The Space–Time Extra-Trees model was used in the CHAP dataset to estimate PM concentrations on the basis of long-term and high spatial resolution aerosol optical depths generated by the Moderate Resolution Imaging Spectroradiometer Multi-Angle Implementation of Atmospheric Correction algorithm (Wei et al. 2021a, b). The Space–Time Extra-Trees model uses several basic information and variables, such as meteorological data (e.g., temperature, humidity, and wind speed), land use/land cover information, and satellite-derived aerosol optical depths, in its training process. These variables were selected on the basis of their known associations with PM concentrations and their availability in the study area. The model’s performance was assessed by using cross-validation and validation against ground-based PM measurements. Strong predictive capabilities with high accuracies were observed in the model. The cross-validation coefficient of determination (CV-R2) for PM2.5 ranged from 0.86 to 0.90, with an average root mean square error ranging from 10.0 to 18.4 μg/m3. With regard to PM10, the dataset showed a high quality, with a cross-validation coefficient of determination ranging from 0.83 to 0.87 and a small root mean square error ranging from 19.7 to 28.4 μg/m3. The CHAP dataset provided daily and monthly concentrations of PM1, PM2.5, PM10, O3, NO2, SO2, and CO, and covered all regions of China from 2000 to 2022 with no missing data.
In this study, the participants’ air pollutant exposure concentrations of ambient PM were estimated through a two-step strategy. In the first step, we geocoded the residence address information of the participants using the application program interface provided by the open platform of Baidu Maps in China (https://lbsyun.baidu.com/). In the second step, we estimated the average exposure concentrations of air pollutants for participants in 250-, 500-, and 750-m diameter circular buffer zones based on each residential location.
Exposure time window
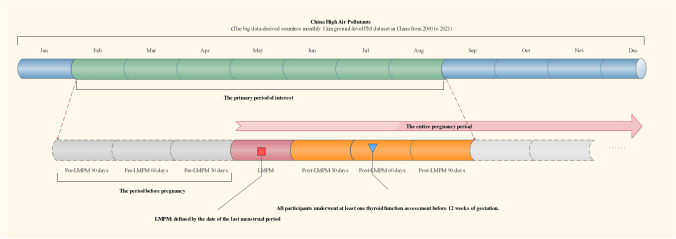
To assess the potential effects of pollutant exposure on hypothyroidism during pregnancy, we defined an exposure window according to the last menstrual period month (LMPM) of pregnant women. The exposure window encompassed the preconception and post-LMPM periods. Preconception exposure was defined as 30, 60, and 90 days preceding the LMPM. Additionally, we assessed pollutant exposure at 30, 60, and 90 days following the LMPM. This post-LMPM exposure assessment enabled us to examine the potential effect of early gestational pollutant exposure on hypothyroidism (Fig. 2).
Fig. 2.
Exposure time window of the study
Statistical analyses
Continuous variables are expressed as the mean (standard deviation), and categorical data are expressed as the number (percentage). Differences in continuous variables between groups were compared using the t-test or Wilcoxon test. Differences in categorical variables between groups were compared using the chi-square test or Fisher’s test.
Propensity score matching was used to match controls using age, ethnicity, gravidity, and parity at a 1:3 ratio. Logistic regression models were used to evaluate the association between airborne PM and hypothyroidism during pregnancy. All logistic regression models were adjusted for age, the season of the last menstruation, and pregnancy and delivery history. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to assess the effect of airborne PM exposure on thyroid disease in pregnancy.
All statistical analyses were performed using R (version 4.1.0, available from: https://www.r-project.org/), and the “MatchIt” package of R was used to perform propensity score matching. The R package “pheatmap” was used to visualize the relationship between ambient PM and hypothyroidism during pregnancy. Quantum Geographical Information System (QGIS, version: 3.26.3) software (available from: http://qgis.osgeo.org) was used to perform spatial analysis and mapping. All P values are two-sided, and significance was set at P < 0.05.
Results
A total of 3,180 women were enrolled in the study, of whom 795 were hypothyroid patients and 2,385 were matched controls. There were no significant differences in ethnicity, age, gravidity, multiparity, newborn sex, birth length, birth weight, or days of gestation between the two groups (Table 1). The proportion of the LMPM in winter in the case group was higher than that in control group (P < 0.05). The geographical distribution of the study participants is shown in Fig. 3. Cases and controls were distributed evenly in Beijing, and most of the study participants were from the Chaoyang District.
Table 1.
Comparison of characteristics of participants in the study between the case and control groups
| Control group (n = 2385) | Case group (n = 795) | P | ||
|---|---|---|---|---|
| Han Chinese (%) | No | 116 (4.9) | 40 (5.0) | 0.924 |
| Yes | 2269 (95.1) | 755 (95.0) | ||
| Age (years) | 31.01 (3.66) | 31.16 (3.71) | 0.320 | |
| Multipara | No | 1926 (80.8) | 657 (82.6) | 0.260 |
| Yes | 459 (19.2) | 138 (17.4) | ||
| Gravidity (times) | 1 | 1476 (61.9) | 474 (59.6) | 0.472 |
| 2 | 568 (23.8) | 196 (24.7) | ||
| > 2 | 341 (14.3) | 125 (15.7) | ||
| Neonatal sex (%) | Male | 1228 (51.5) | 424 (53.3) | 0.389 |
| Female | 1157 (48.5) | 371 (46.7) | ||
| Neonatal length (cm) | 50.58 (2.36) | 50.48 (2.38) | 0.296 | |
| Birth weight (g) | 3297.18 (486.74) | 3285.73 (482.09) | 0.565 | |
| LMPM season (%) | Spring | 430 (18.0) | 209 (26.3) | < 0.001 |
| Summer | 755 (31.7) | 195 (24.5) | ||
| Autumn | 763 (32.0) | 175 (22.0) | ||
| Winter | 437 (18.3) | 216 (27.2) | ||
| Days of gestation (days) | 276.22 (12.07) | 276.58 (12.76) | 0.466 | |
| TSH (uIU/mL) | 1.72 (0.69) | 5.22 (0.75) | < 0.001 | |
| FT4 (ng/dL) | 1.32 (0.13) | 0.71 (0.15) | < 0.001 |
LMPM: last menstrual period month; TSH: thyroid-stimulating hormone; FT4: free thyroxine; n: number of participants
Fig. 3.
Geographical distribution of pregnant women included in the study. The controls are shown as green dots and the cases are shown as red stars
The differences in preconception and early pregnancy exposure to PM2.5 and PM10 between the two groups of pregnant women at 250-, 500-, and 750-m buffer distances are shown in Table 2. The average concentration of PM10 was significantly higher in the case group compared to the control group at 30 days pre-LMPM, the LMPM, and 30 days post-LMPM for all three buffer distances (P < 0.05). Furthermore, the case group exhibited significantly higher average PM2.5 concentrations compared to the control group at 60 days pre-LMPM, 30 days pre-LMPM, the LMPM, and 30 days post-LMPM for all three buffer distances (P < 0.05).
Table 2.
Pregnant women exposed to PM2.5 at 250, 500, and 750 m buffer distances at preconception and in early pregnancy
| PM2.5 | PM10 | |||||
|---|---|---|---|---|---|---|
| Control group | Case group | P | Control group | Case group | P | |
| 250 m buffer | ||||||
| Pre-LMPM 90 days | 69.63 (29.67) | 68.86 (28.37) | 0.526 | 110.03 (36.15) | 106.67 (35.22) | 0.023 |
| Pre-LMPM 60 days | 67.09 (27.39) | 70.10 (30.66) | 0.009* | 107.35 (35.32) | 109.15 (37.75) | 0.220 |
| Pre-LMPM 30 days | 62.78 (23.72) | 67.47 (29.50) | < 0.001* | 102.28 (31.41) | 106.10 (36.13) | 0.004 |
| LMPM | 58.37 (19.14) | 67.04 (28.69) | < 0.001* | 95.79 (26.78) | 105.50 (35.66) | < 0.001 |
| Post-LMPM 30 days | 62.19 (23.24) | 66.13 (26.74) | < 0.001* | 99.29 (30.71) | 104.45 (33.51) | < 0.001 |
| Post-LMPM 60 days | 65.04 (27.21) | 66.28 (28.63) | 0.275 | 101.91 (34.32) | 104.63 (35.72) | 0.055 |
| Post-LMPM 90 days | 64.45 (28.05) | 65.23 (27.53) | 0.499 | 100.92 (34.65) | 103.27 (34.70) | 0.098 |
| 500 m buffer | ||||||
| Pre-LMPM 90 days | 69.54 (29.60) | 68.81 (28.30) | 0.545 | 110.01 (36.09) | 106.67 (35.13) | 0.023 |
| Pre-LMPM 60 days | 67.00 (27.33) | 70.03 (30.59) | 0.009* | 107.34 (35.27) | 109.15 (37.66) | 0.220 |
| Pre-LMPM 30 days | 62.70 (23.70) | 67.38 (29.37) | < 0.001* | 102.28 (31.42) | 106.09 (36.01) | 0.004* |
| LMPM | 58.30 (19.09) | 66.95 (28.57) | < 0.001* | 95.79 (26.76) | 105.43 (35.47) | < 0.001* |
| Post-LMPM 30 days | 62.09 (23.18) | 66.02 (26.63) | < 0.001* | 99.29 (30.67) | 104.40 (33.34) | < 0.001* |
| Post-LMPM 60 days | 64.95 (27.14) | 66.18 (28.55) | 0.274 | 101.92 (34.29) | 104.65 (35.63) | 0.054 |
| Post-LMPM 90 days | 64.37 (27.97) | 65.14 (27.48) | 0.502 | 100.93 (34.59) | 103.29 (34.68) | 0.097 |
| 750 m buffer | ||||||
| Pre-LMPM 90 days | 69.44 (29.51) | 68.73 (28.23) | 0.550 | 110.09 (36.06) | 106.75 (35.06) | 0.023 |
| Pre-LMPM 60 days | 66.90 (27.27) | 69.95 (30.53) | 0.008* | 107.42 (35.23) | 109.22 (37.60) | 0.221 |
| Pre-LMPM 30 days | 62.60 (23.65) | 67.28 (29.23) | < 0.001* | 102.36 (31.44) | 106.14 (35.90) | 0.005* |
| LMPM | 58.23 (19.03) | 66.84 (28.42) | < 0.001* | 95.89 (26.74) | 105.45 (35.30) | < 0.001* |
| Post-LMPM 30 days | 62.00 (23.12) | 65.90 (26.51) | < 0.001* | 99.39 (30.64) | 104.44 (33.23) | < 0.001* |
| Post-LMPM 60 days | 64.85 (27.06) | 66.08 (28.45) | 0.275 | 102.03 (34.26) | 104.73 (35.52) | 0.057 |
| Post-LMPM 90 days | 64.28 (27.88) | 65.02 (27.40) | 0.518 | 101.02 (34.52) | 103.33 (34.59) | 0.103 |
LMPM: last menstrual period month. the units of ambient particulate matter were μg/m3. *P < 0.05
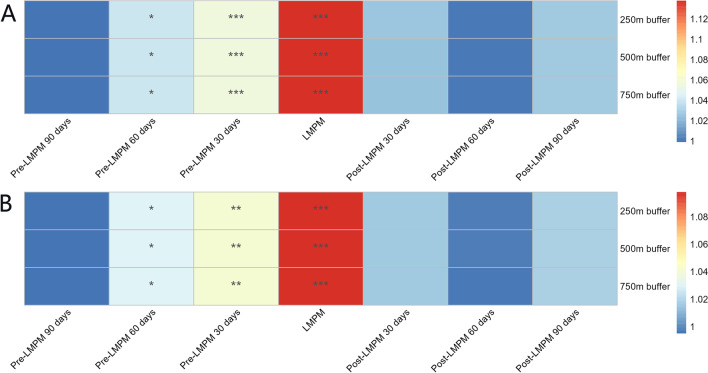
Table 3 shows the logistic regression results of the association between hypothyroidism during pregnancy and air pollution, such as PM2.5 and PM10. Significant differences in the ORs of PM2.5 and PM10 were found at 60 days pre-LMPM, 30 days pre-LMPM 30 days, and the LMPM for all three distance buffers. No significant differences were found in the ORs of PM2.5 or PM10 during the post-LMPM periods for any distance buffer. Furthermore, the strongest significant difference in PM2.5 and PM10 was observed during the LMPM period across all distance buffers, with ORs of 1.137 (95% CI: 1.096–1.180) and 1.098 (95% CI: 1.067–1.130), respectively. The ORs at 30 days pre-LMPM were also significantly different from 1 for all distance buffers in both models, indicating that this time could also be important for the studied association. In addition, the ORs of PM10 and PM2.5 at 90 days pre-LMPM were not significantly different from 1 for all distance buffers. Overall, these results suggested that the LMPM period and the 30-day pre-LMPM period were the most important time periods for the association between hypothyroidism during pregnancy and air pollution. The heat map of OR values for logistic regression of ambient PM and hypothyroidism in pregnancy is shown in Fig. 4.
Table 3.
Relationships between hypothyroidism during pregnancy and exposure to PM2.5 and PM10
| PM2.5 | PM10 | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| 250 m buffer | ||||
| Pre-LMPM 90 days | 0.999(0.968–1.030) | 0.937 | 0.995(0.970–1.021) | 0.716 |
| Pre-LMPM 60 days | 1.040(1.008–1.072) | 0.012* | 1.030(1.004–1.055) | 0.021* |
| Pre-LMPM 30 days | 1.056(1.023–1.090) | < 0.001* | 1.041(1.013–1.070) | 0.004* |
| LMPM | 1.137(1.096–1.180) | < 0.001* | 1.098(1.067–1.130) | < 0.001* |
| Post-LMPM 30 days | 1.026(0.991–1.062) | 0.150 | 1.016(0.989–1.044) | 0.241 |
| Post-LMPM 60 days | 1.001(0.971–1.033) | 0.930 | 0.998(0.973–1.023) | 0.868 |
| Post-LMPM 90 days | 1.028(0.994–1.062) | 0.100 | 1.018(0.992–1.046) | 0.179 |
| 500 m buffer | ||||
| Pre-LMPM 90 days | 0.999(0.969–1.031) | 0.966 | 0.996(0.971–1.021) | 0.734 |
| Pre-LMPM 60 days | 1.040(1.009–1.072) | 0.012* | 1.030(1.005–1.056) | 0.020* |
| Pre-LMPM 30 days | 1.056(1.023–1.090) | < 0.001* | 1.041(1.013–1.070) | 0.004* |
| LMPM | 1.138(1.097–1.181) | < 0.001* | 1.098(1.066–1.130) | < 0.001* |
| Post-LMPM 30 days | 1.025(0.990–1.061) | 0.157 | 1.016(0.989–1.044) | 0.254 |
| Post-LMPM 60 days | 1.001(0.970–1.033) | 0.937 | 0.998(0.973–1.023) | 0.864 |
| Post-LMPM 90 days | 1.028(0.994–1.063) | 0.102 | 1.018(0.991–1.046) | 0.181 |
| 750 m buffer | ||||
| Pre-LMPM 90 days | 1.000(0.969–1.031) | 0.978 | 0.996(0.971–1.021) | 0.737 |
| Pre-LMPM 60 days | 1.040(1.009–1.073) | 0.011* | 1.030(1.005–1.056) | 0.020* |
| Pre-LMPM 30 days | 1.056(1.023–1.090) | < 0.001* | 1.041(1.013–1.070) | 0.004* |
| LMPM | 1.138(1.097–1.181) | < 0.001* | 1.098(1.066–1.130) | < 0.001* |
| Post-LMPM 30 days | 1.025(0.990–1.061) | 0.165 | 1.016(0.988–1.044) | 0.270 |
| Post-LMPM 60 days | 1.001(0.970–1.033) | 0.950 | 0.997(0.972–1.023) | 0.843 |
| Post-LMPM 90 days | 1.028(0.994–1.062) | 0.108 | 1.018(0.991–1.045) | 0.191 |
LMPM: last menstrual period month.; OR (95% CI): odds ratio (95% confidence interval); OR values represent the increased risk of hypothyroidism per 10 µg/m3 of ambient particulate matter exposure. *P < 0.05. All logistic regression models were adjusted for age, the season of the last menstruation, and pregnancy and delivery history
Fig. 4.
Heat map of OR values for logistic regression of ambient particulate matter and hypothyroidism. LMP: last menstrual period. A: ORs of logistic regression of PM2.5 and hypothyroidism; B: ORs of logistic regression of PM10 and hypothyroidism. OR values represent the increased risk of hypothyroidism per 10 µg/m.3 of ambient particulate matter exposure. *P < 0.05; **P < 0.01 ***P < 0.001
Table 4 shows the subgroup analysis of the relationship between hypothyroidism during pregnancy and air pollution in the population of Changping District. The results of the subgroup analysis were consistent with the results of the main analysis. Significant differences in the ORs for PM2.5 and PM10 at 60 days pre-LMPM, 30 days pre-LMPM 30 days, and the LMPM were found at all three distance buffers. No significant differences were found in the ORs during the post-LMPM periods for any distance buffer. The strongest significant difference in PM2.5was observed during the LMPM at the 750-m buffer (OR: 1.140, 95% CI: 1.088–1.196). The strongest significant difference in PM10 was observed during the LMPM at the 250-m buffer (OR: 1.100, 95% CI: 1.060–1.141).
Table 4.
Relationships between hypothyroidism during pregnancy and exposure to PM2.5 and PM10 in Changping District
| PM2.5 | PM10 | |||
|---|---|---|---|---|
| OR | P | OR | P | |
| 250 m buffer | ||||
| Pre-LMPM 90 days | 1.012(0.973–1.052) | 0.561 | 1.005(0.973–1.037) | 0.771 |
| Pre-LMPM 60 days | 1.052(1.012–1.093) | 0.009* | 1.042(1.009–1.075) | 0.011* |
| Pre-LMPM 30 days | 1.070(1.029–1.113) | < 0.001* | 1.053(1.019–1.090) | 0.002* |
| LMPM | 1.139(1.088–1.194) | < 0.001* | 1.100(1.060–1.141) | < 0.001* |
| Post-LMPM 30 days | 1.038(0.995–1.083) | 0.086 | 1.025(0.990–1.060) | 0.160 |
| Post-LMPM 60 days | 1.008(0.971–1.047) | 0.666 | 1.006(0.975–1.038) | 0.702 |
| Post-LMPM 90 days | 1.038(0.996–1.082) | 0.072 | 1.030(0.997–1.065) | 0.077 |
| 500 m buffer | ||||
| Pre-LMPM 90 days | 1.012(0.973–1.053) | 0.538 | 1.005(0.973–1.038) | 0.769 |
| Pre-LMPM 60 days | 1.052(1.013–1.093) | 0.009* | 1.042(1.009–1.075) | 0.012* |
| Pre-LMPM 30 days | 1.070(1.029–1.113) | < 0.001* | 1.054(1.019–1.090) | 0.002* |
| LMPM | 1.140(1.088–1.195) | < 0.001* | 1.099(1.059–1.141) | < 0.001* |
| Post-LMPM 30 days | 1.038(0.994–1.083) | 0.090 | 1.025(0.990–1.060) | 0.164 |
| Post-LMPM 60 days | 1.008(0.970–1.047) | 0.670 | 1.006(0.975–1.038) | 0.702 |
| Post-LMPM 90 days | 1.038(0.996–1.082) | 0.072 | 1.030(0.996–1.065) | 0.080 |
| 750 m buffer | ||||
| Pre-LMPM 90 days | 1.013(0.973–1.053) | 0.532 | 1.005(0.973–1.038) | 0.772 |
| Pre-LMPM 60 days | 1.053(1.013–1.094) | 0.009* | 1.042(1.009–1.075) | 0.012* |
| Pre-LMPM 30 days | 1.071(1.029–1.114) | < 0.001* | 1.053(1.018–1.089) | 0.003* |
| LMPM | 1.140(1.088–1.196) | < 0.001* | 1.099(1.059–1.140) | < 0.001* |
| Post-LMPM 30 days | 1.037(0.994–1.083) | 0.094 | 1.024(0.990–1.060) | 0.171 |
| Post-LMPM 60 days | 1.008(0.970–1.047) | 0.685 | 1.006(0.974–1.038) | 0.722 |
| Post-LMPM 90 days | 1.038(0.996–1.082) | 0.078 | 1.030(0.996–1.065) | 0.085 |
LMPM: last menstrual period month.; OR (95% CI): odds ratio (95% confidence interval). OR values represent the increased risk of hypothyroidism per 10 µg/m3 of ambient particulate matter exposure. *P < 0.05. All logistic regression models were adjusted for age, the season of the last menstruation, and pregnancy and delivery history
Discussion
This study investigated the association between preconception and early pregnancy exposure to PM and the risk of hypothyroidism during pregnancy. Using a retrospective case–control study design, in combination with the highest resolution exposure data available in the Chinese region, we found that PM at 60 days before conception and at the month of conception was a risk factor for hypothyroidism during pregnancy. The subgroup analysis and buffering zone analysis with different ranges further confirmed the stability of our results.
Previous studies have associated long-term exposure to air pollutants with diseases, such as cardiovascular disease, lung disease, and adverse reproductive outcomes (Boogaard et al. 2022; Franklin et al. 2015; Yang et al. 2021; Zhou et al. 2022a). In this study, we found that exposure to PM in the first month of pregnancy was a risk factor for hypothyroidism during pregnancy, which is consistent with previous studies (Ghassabian et al. 2019; Zhang et al. 2022; Zhao et al. 2019). Zhao et al. showed that PM exposure during early and mid-pregnancy was a risk factor for hypothyroidism during pregnancy (Zhao et al. 2019). Furthermore, another study showed an association between early pregnancy exposure to PM2.5 and reduced FT4 concentrations, which are an indicator of hypothyroidism (Zhang et al. 2022). A large European population cohort study showed that higher exposure to PM2.5 was associated with a higher risk of hypothyroxinemia, but exposure to PM10 was not significantly associated with hypothyroxinemia (Ghassabian et al. 2019). The above-mentioned studies established a relationship between ambient air pollution exposure during early pregnancy and hypothyroidism. However, limited research has investigated the association between air pollution exposure during preconception and hypothyroidism.
In the present study, we found that exposure to air pollution during the LMP, and at 30 and 60 days before the conception month was a risk factor for hypothyroidism. The preconception period is a crucial stage in pregnancy. Good preconception care can reduce the incidence of fetal malformations and improve the health of the fetus (Berghella et al. 2010). A growing number of studies have shown associations between preconception air pollution exposure and maternal health and fetal outcomes during pregnancy. One study showed an association between preconception air pollutant exposure and the risk of congenital heart disease in newborns (Vecoli et al. 2016). A meta-analysis of 31 cohort studies showed that exposure to PM2.5 and PM10 was associated with an increased risk of gestational diabetes, especially at preconception and the first trimester of pregnancy (Liang et al. 2023). A systematic review showed that maternal pre-pregnancy exposure to PM2.5 and PM10 was associated with child health, including birth defects, preterm birth, low birth weight, and autism (Blanc et al. 2023). Therefore, additional research is required to validate the association between preconception exposure to air pollution and hypothyroidism. Nevertheless, because air pollution exposure is a modifiable risk factor, it should be avoided before conception.
The mechanisms underlying the effect of preconception exposure to environmental PM on hypothyroidism remain poorly understood. Potential mechanisms may involve genetic factors, oxidative stress, inflammation, and metabolic dysregulation. Previous studies have suggested genetic involvement in the association between environmental exposures and disease (Moubarz et al. 2023; Saad-Hussein et al. 2022). Exposure to environmental PM can affect thyroid function by modulating gene expression and signaling pathways associated with the thyroid (Wen et al. 2021). Additionally, individual genetic variations, such as gene polymorphisms, may affect the sensitivity and metabolic capacity toward environmental PM, thereby contributing to individual differences in the effects on thyroid function (Huang et al. 2004). Inflammation is one possible mechanism of PM exposure because environmental PM can enter the body through the respiratory system, leading to an association with inflammatory responses and potential effects on thyroid function (Jiang et al. 2021; Liu et al. 2022). Another potential mechanism is endocrine disruption in which certain components of air pollution, such as polycyclic aromatic hydrocarbons, possess endocrine-disrupting properties that interfere with the normal functioning of the thyroid gland (Darbre 2018; Dehghani et al. 2022). Additionally, oxidative stress is considered another potential mechanism because air pollutants have the ability to generate reactive oxygen species and induce oxidative stress, resulting in damage to thyroid tissue and impairment of thyroid hormone synthesis and function (Chen et al. 2023). Further research is required to gain a deeper understanding of these mechanisms and their interplay to determine the complex relationship between preconception environmental PM exposure and thyroid dysfunction.
In our study, no significant association was found between air pollution exposure from weeks 4 to 18 of pregnancy and hypothyroidism, which is inconsistent with a previous study (Zhang et al. 2022). One possible reason for this outcome is that our participants’ outcomes were defined in the early stages of pregnancy (before 12 weeks’ gestation). Therefore, we cannot ascertain whether PM exposure from weeks 4 to 18 of pregnancy occurs before the onset of hypothyroidism. Consequently, inferring a causal relationship between environmental PM exposure and hypothyroidism is difficult. This issue may be one of the factors contributing to the observed disparity in results between studies. In this study, different buffer distances of 250, 500, and 750 m were used to accurately assess the exposure to air pollutants in pregnant women. The buffer analysis was used to establish buffer zones at various distances to investigate air pollutant concentrations at different ranges, which is a method extensively used in previous studies. (Graafland et al. 2023; Mendrinos et al. 2022). In our study, differences in PM10 and PM2.5 between the groups were observed at all three buffer zones, which indicates the stability of our results.
Overall, the present study provides considerable insight into the health risks associated with air pollution exposure during preconception and early pregnancy, emphasizing the necessity for effective interventions to mitigate exposure to air pollutants and protect maternal well-being. Nonetheless, we need to acknowledge the inherent limitations to our study. First, our study was conducted at a single center, necessitating further validation and investigation before generalizing the findings to broader populations or domains. Subsequent investigations should use a multicenter, multi-regional study design. Second, the assessment of indoor pollution was not performed when measuring PM exposure. Consequently, future studies should comprehensively examine the association between indoor and outdoor pollution exposure and decreased thyroid function during pregnancy. Third, the sample size in this study was relatively small. To avoid ecological fallacies, the study population was limited to the Beijing area, with a subgroup analysis conducted on the Chaoyang District population, which demonstrated the robustness of the findings. Fourth, the number of positive samples in this study was limited, and propensity score matching was used taking into consideration factors, such as maternal age, ethnicity, the number of pregnancies, and the number of births, to minimize selection bias in control selection. However, certain confounding factors, such as smoking and alcohol consumption habits, were not accounted for in our study. A previous study showed an association between smoking behavior and elevated concentrations of thyroid hormones (Gruppen et al. 2020). Therefore, future studies should consider the effect of these confounding factors on the outcomes.
Conclusions
This study shows that exposure to PM2.5 and PM10 during the preconception and early pregnancy periods poses a considerable risk of thyroid disease in pregnancy, particularly on exposure during the month of conception. These results highlight the urgent requirement for comprehensive strategies to address air pollution and protect maternal thyroid health. Policymakers and healthcare professionals should prioritize interventions aimed at reducing air pollution levels and promoting awareness regarding its detrimental effects on pregnancy outcomes. Further research is warranted to determine the underlying mechanisms and long-term implications of air pollution on thyroid health in pregnancy. This study provides important insight that contributes to the growing body of evidence on the association of air pollution and maternal health. This evidence suggests the importance of implementing targeted interventions and policies to mitigate the adverse effects of air pollution on pregnant women.
Acknowledgements
We thank all participants in this study
Author contributions
Qi Zhang and Qi Sun contributed to the conception and design of the study. Data collection was performed by Yuanmei Chen, Fang Ye, and Jing Liu. Data analysis was performed by Qi Sun, Liu Fei, and Hui Qin. The first draft of the manuscript was by Qi Sun, and all authors contributed to the revision of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by MOE Key Laboratory of Population Health Across Life Cycle (Grant numbers: JK20225) and Chinese Academy of Medical Sciences Clinical and Translational Medicine Research Project (Grant numbers: 2021-I2M-C&T-B-089).
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
The study was conducted under the ethical approval of the Ethical Review Board in China-Japan Friendship Hospital (No: 2022-KY-006–1).
Consent to participate
The data are anonymous, and the requirement for informed consent was therefore waived.
Consent to publish
All authors approve the manuscript and give their consent for submission and publication.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bai W, Li Y, Niu Y, Ding Y, Yu X, Zhu B, Duan R, Duan H, Kou C, Li Y. Association between ambient air pollution and pregnancy complications: a systematic review and meta-analysis of cohort studies. Environ Res. 2020;185:109471. doi: 10.1016/j.envres.2020.109471. [DOI] [PubMed] [Google Scholar]
- Berghella V, Buchanan E, Pereira L, Baxter JK. Preconception Care. Obstetrical & Gynecological Survey. 2010;65:119–131. doi: 10.1097/OGX.0b013e3181d0c358. [DOI] [PubMed] [Google Scholar]
- Blanc N, Liao J, Gilliland F, Zhang JJ, Berhane K, Huang G, Yan W, Chen Z. A systematic review of evidence for maternal preconception exposure to outdoor air pollution on Children’s health. Environ Pollut (Barking, Essex: 1987) 2023;318:120850. doi: 10.1016/j.envpol.2022.120850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogaard H, Patton AP, Atkinson RW, Brook JR, Chang HH, Crouse DL, Fussell JC, Hoek G, Hoffmann B, Kappeler R. Long-term exposure to traffic-related air pollution and selected health outcomes: A systematic review and meta-analysis. Environ Int. 2022;164:107262. doi: 10.1016/j.envint.2022.107262. [DOI] [PubMed] [Google Scholar]
- Cao L, Wang L, Wu L, Wang T, Cui X, Yu L, Diao R, Mao H. Particulate matter and hypertensive disorders in pregnancy: systematic review and meta-analysis. Public Health. 2021;200:22–32. doi: 10.1016/j.puhe.2021.08.013. [DOI] [PubMed] [Google Scholar]
- Chaker L, Razvi S, Bensenor IM, Azizi F, Pearce EN, Peeters RP. Hypothyroidism. Nat Rev Dis Primers. 2022;8:30. doi: 10.1038/s41572-022-00357-7. [DOI] [PubMed] [Google Scholar]
- Chen L, Li H, Ru Y, Song Y, Shen Y, Zhao L, Huang G, Chen Y, Qi Z, Li R. Xanthine-derived reactive oxygen species exacerbates adipose tissue disorders in male db/db mice induced by real-ambient PM2. 5 exposure. Sci Total Environ. 2023;882:163592. doi: 10.1016/j.scitotenv.2023.163592. [DOI] [PubMed] [Google Scholar]
- Darbre PD. Overview of air pollution and endocrine disorders. Int J Gen Med. 2018;11:191–207. doi: 10.2147/IJGM.S102230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani MH, Hopke PK, Asghari FB, Mohammadi AA, Yousefi M. The effect of the decreasing level of Urmia Lake on particulate matter trends and attributed health effects in Tabriz. Iran Microchem J. 2020;153:104434. doi: 10.1016/j.microc.2019.104434. [DOI] [Google Scholar]
- Dehghani S, Fararouei M, Rafiee A, Hoepner L, Oskoei V, Hoseini M. Prenatal exposure to polycyclic aromatic hydrocarbons and effects on neonatal anthropometric indices and thyroid-stimulating hormone in a Middle Eastern population. Chemosphere. 2022;286:131605. doi: 10.1016/j.chemosphere.2021.131605. [DOI] [PubMed] [Google Scholar]
- DiSantostefano J. International classification of diseases 10th revision (ICD-10) J Nurse Practitioners. 2009;5:56–57. doi: 10.1016/j.nurpra.2008.09.020. [DOI] [Google Scholar]
- Eshkoli T, Wainstock T, Sheiner E, Beharier O, Fraenkel M, Walfisch A. Maternal Hypothyroidism during Pregnancy and the Risk of Pediatric Endocrine Morbidity in the Offspring. Am J Perinatol. 2019;36:975–980. doi: 10.1055/s-0038-1675834. [DOI] [PubMed] [Google Scholar]
- Franklin BA, Brook R, Arden Pope C., 3rd Air pollution and cardiovascular disease. Curr Probl Cardiol. 2015;40:207–238. doi: 10.1016/j.cpcardiol.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Ghassabian A, et al. Association of Exposure to Ambient Air Pollution With Thyroid Function During Pregnancy. JAMA Netw Open. 2019;2:e1912902. doi: 10.1001/jamanetworkopen.2019.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graafland N, Essers E, Posthumus A, Gootjes D, Ambrós A, Steegers E, Guxens M. Exposure to outdoor residential noise during pregnancy, embryonic size, fetal growth, and birth outcomes. Environ Int. 2023;171:107730. doi: 10.1016/j.envint.2023.107730. [DOI] [PubMed] [Google Scholar]
- Gruppen EG, Kootstra-Ros J, Kobold AM, Connelly MA, Touw D, Bos JHJ, Hak E, Links TP, Bakker SJL, Dullaart RPF. Cigarette smoking is associated with higher thyroid hormone and lower TSH levels: the PREVEND study. Endocrine. 2020;67:613–622. doi: 10.1007/s12020-019-02125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Tsai P-C, Guo YL. Multiple gene polymorphisms as human susceptibility factors to multiple outcomes of persistent organic pollutants (POPS) Epidemiology. 2004;15:S73–S74. doi: 10.1097/00001648-200407000-00181. [DOI] [Google Scholar]
- Huget-Penner S, Feig DS. Maternal thyroid disease and its effects on the fetus and perinatal outcomes. Prenat Diagn. 2020;40:1077–1084. doi: 10.1002/pd.5684. [DOI] [PubMed] [Google Scholar]
- Jiang X, Han Y, Qiu X, Chai Q, Zhang H, Chen X, Cheng Z, Wang Y, Fan Y, Xue T. Organic components of personal PM2. 5 exposure associated with inflammation: Evidence from an untargeted exposomic approach. Environ Sci Technol. 2021;55:10589–10596. doi: 10.1021/acs.est.1c02023. [DOI] [PubMed] [Google Scholar]
- Korevaar TIM, Medici M, Visser TJ, Peeters RP. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol. 2017;13:610–622. doi: 10.1038/nrendo.2017.93. [DOI] [PubMed] [Google Scholar]
- Korevaar TIM, et al. Association of Thyroid Function Test Abnormalities and Thyroid Autoimmunity With Preterm Birth: A Systematic Review and Meta-analysis. JAMA. 2019;322:632–641. doi: 10.1001/jama.2019.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Zhu H, Xu J, Zhao Z, Zhou L, Zhu Q, Cai J, Ji L. Ambient air pollution and gestational diabetes mellitus: An updated systematic review and meta-analysis. Ecotoxicol Environ Saf. 2023;255:114802. doi: 10.1016/j.ecoenv.2023.114802. [DOI] [PubMed] [Google Scholar]
- Liu C, Yang J, Du X, Geng X. Filtered air intervention modulates hypothalamic-pituitary-thyroid/gonadal axes by attenuating inflammatory responses in adult rats after fine particulate matter (PM2. 5) exposure. Environ Sci Pollut Res. 2022;29:74851–74860. doi: 10.1007/s11356-022-21102-3. [DOI] [PubMed] [Google Scholar]
- Maraka S, Ospina NM, O'Keeffe DT, Espinosa De Ycaza AE, Gionfriddo MR, Erwin PJ, Coddington CC, 3rd, Stan MN, Murad MH, Montori VM. Subclinical Hypothyroidism in Pregnancy: A Systematic Review and Meta-Analysis. Thyroid. 2016;26:580–590. doi: 10.1089/thy.2015.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici M, Korevaar TIM, Visser WE, Visser TJ, Peeters RP. Thyroid Function in Pregnancy: What Is Normal? Clin Chem. 2015;61:704–713. doi: 10.1373/clinchem.2014.236646. [DOI] [PubMed] [Google Scholar]
- Mendrinos A, Ramesh B, Ruktanonchai CW, Gohlke JM. Poultry concentrated animal-feeding operations on the Eastern Shore, Virginia, and geospatial associations with adverse birth outcomes. Healthcare (Basel, Switzerland) 2022;10:2016. doi: 10.3390/healthcare10102016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moubarz G, Mohammed AMF, Saleh IA, Shahy EM, Helmy MA. Nephrotoxic effect of heavy metals and the role of DNA repair gene among secondary aluminum smelter workers. Environ Sci Pollut Res Int. 2023;30:29814–29823. doi: 10.1007/s11356-022-24270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellano P, Reynoso J, Quaranta N, Bardach A, Ciapponi A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ Int. 2020;142:105876. doi: 10.1016/j.envint.2020.105876. [DOI] [PubMed] [Google Scholar]
- Ross DS (2013) Hypothyroidism during pregnancy: clinical manifestations, diagnosis, and treatment. UpToDate. Waltham, MA. https://www.uptodate.com/contents/hypothyroidism-during-pregnancy-clinical-manifestations-diagnosis-and-treatment. Accessed 8 Jun 2013
- Saad-Hussein A, Moubarz G, Mahdy-Abdallah H, Helmy MA. Impact of mannose-binding lectin gene polymorphism on lung functions among workers exposed to airborne Aspergillus in a wastewater treatment plant in Egypt. Environ Sci Pollut Res Int. 2022;29:63193–63201. doi: 10.1007/s11356-022-20234-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma-Pillay P, Nelson-Piercy C, Tolppanen H, Mebazaa A. Physiological changes in pregnancy: review articles. Cardiovasc J Afr. 2016;27:89–94. doi: 10.5830/CVJA-2016-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Liu Y, Li G, Liu X, Huang S, Duan T, Du Q. Associations of Hypothyroxinemia With Risk of Preeclampsia-Eclampsia and Gestational Hypertension. Front Endocrinol. 2021;12:777152. doi: 10.3389/fendo.2021.777152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecoli C, Pulignani S, Andreassi MG. Genetic and epigenetic mechanisms linking air pollution and congenital heart disease. J Cardiovasc Develop Dis. 2016;3:32. doi: 10.3390/jcdd3040032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu C, Zhang M, Han Y, Aase H, Villanger GD, Myhre O, Donkelaar AV, Martin RV, Baines EA, Chen R, Kan H, Xia Y. Evaluation of Maternal Exposure to PM(2.5) and Its Components on Maternal and Neonatal Thyroid Function and Birth Weight: A Cohort Study. Thyroid. 2019;29:1147–1157. doi: 10.1089/thy.2018.0780. [DOI] [PubMed] [Google Scholar]
- Wang L, Luo D, Liu X, Zhu J, Wang F, Li B, Li L. Effects of PM2 5 exposure on reproductive system and its mechanisms. Chemosphere. 2021;264:128436. doi: 10.1016/j.chemosphere.2020.128436. [DOI] [PubMed] [Google Scholar]
- Wei J, Li Z, Lyapustin A, Sun L, Peng Y, Xue W, Su T, Cribb M. Reconstructing 1-km-resolution high-quality PM2.5 data records from to 2018 in China: spatiotemporal variations and policy implications. Remote Sens Environ. 2021;252:112136. doi: 10.1016/j.rse.2020.112136. [DOI] [Google Scholar]
- Wei J, Li Z, Xue W, Sun L, Fan T, Liu L, Su T, Cribb M. The ChinaHighPM10 dataset: generation, validation, and spatiotemporal variations from 2015 to 2019 across China. Environ Int. 2021;146:106290. doi: 10.1016/j.envint.2020.106290. [DOI] [PubMed] [Google Scholar]
- Wen Y, Ding X, Guan Q, Hu W, Wang B, Hu Q, Bigambo FM, Zhou Z, Wang X, Xia Y. Effects of exposure to urban particulate matter SRM 1648a during pregnancy on the neurobehavioral development of offspring mice. Ecotoxicol Environ Saf. 2021;215:112142. doi: 10.1016/j.ecoenv.2021.112142. [DOI] [PubMed] [Google Scholar]
- Yang T, et al. Association of fine particulate matter air pollution and its constituents with lung function: The China Pulmonary Health study. Environ Int. 2021;156:106707. doi: 10.1016/j.envint.2021.106707. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang H, Pan X, Teng W, Shan Z. Patients with subclinical hypothyroidism before 20 weeks of pregnancy have a higher risk of miscarriage: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0175708. doi: 10.1371/journal.pone.0175708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang Q, He S, Wu K, Ren M, Dong H, Di J, Yu Z, Huang C. Ambient air pollution and gestational diabetes mellitus: A review of evidence from biological mechanisms to population epidemiology. Sci Total Environ. 2020;719:137349. doi: 10.1016/j.scitotenv.2020.137349. [DOI] [PubMed] [Google Scholar]
- Zhang X, Huels A, Makuch R, Zhou A, Zheng T, Xia W, Gaskins A, Makuch J, Zhu Z, Zhu C, Qian Z, Xu S, Li Y. Association of exposure to ambient particulate matter with maternal thyroid function in early pregnancy. Environ Res. 2022;214:113942. doi: 10.1016/j.envres.2022.113942. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Cao Z, Li H, Su X, Yang Y, Liu C, Hua J. Air pollution exposure in association with maternal thyroid function during early pregnancy. J Hazard Mater. 2019;367:188–193. doi: 10.1016/j.jhazmat.2018.12.078. [DOI] [PubMed] [Google Scholar]
- Zhou G, Wu J, Yang M, Sun P, Gong Y, Chai J, Zhang J, Afrim F-K, Dong W, Sun R. Prenatal exposure to air pollution and the risk of preterm birth in rural population of Henan Province. Chemosphere. 2022;286:131833. doi: 10.1016/j.chemosphere.2021.131833. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhu Q, Wang P, Li J, Luo R, Zhao W, Zhang L, Shi H, Zhang Y. Early pregnancy PM(25) exposure and its inorganic constituents affect fetal growth by interrupting maternal thyroid function. Environ Pollut (Barking, Essex 1987) 2022;307:119481. doi: 10.1016/j.envpol.2022.119481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.