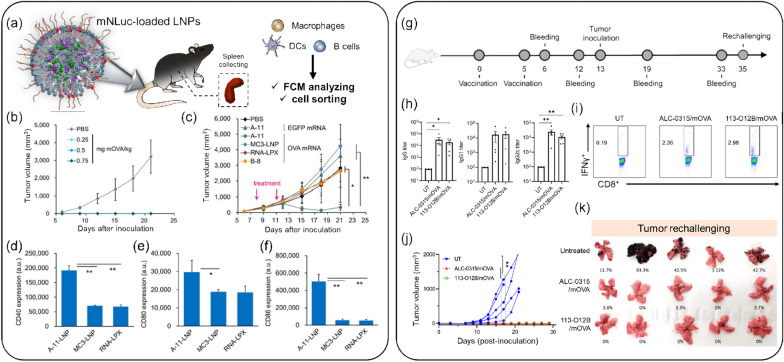
Fig. 4.
Representative mRNA-LNPs for cancer vaccines. a Schematic illustration of the mRNA-loaded LNPs and the experimental method employed. b Prophylactic antitumor activity of A11-LNPs in E.G7-OVA tumor-bearing mice. c Therapeutic antitumor activity of A-11-LNPs, MC3-LNP, RNA-LPX, and B-8-LNPs in E.G7-OVA tumor-bearing mice, intravenously (i.v.) injected with OVA mRNA-loaded formulations at two doses of 0.03 mg mRNA/kg on days 8 and 11 (n = 5). d–f Expression of activation markers CD40 (d), CD80 (e), and CD86 (f) in splenic dendritic cells (DCs) 24 h after an i.v. injection of OVA mRNA-loaded formulations at a dose of 0.03 mg mRNA/kg (n = 3). (* p < 0.05, ** p < 0.01). a–f: Reproduced from a previous report [70] with Elsevier.) (g) Experimental timeline for vaccination and blood withdrawal. h OVA-specific antibody titers in mice treated with 113-O12B/mOVA and ALC-0315/mOVA on day 12. (i) Representative flow cytometry diagrams of IFN-γ-positive cells within CD3 + CD8 + T cells 7 days after the second vaccination. j Tumor volumes in the B16F10-OVA tumor model. (k) Lungs collected 18 days after the i.v. injection of B16F10-OVA cells. g–k: Reproduced from a previous report [72] with permission from PNAS)