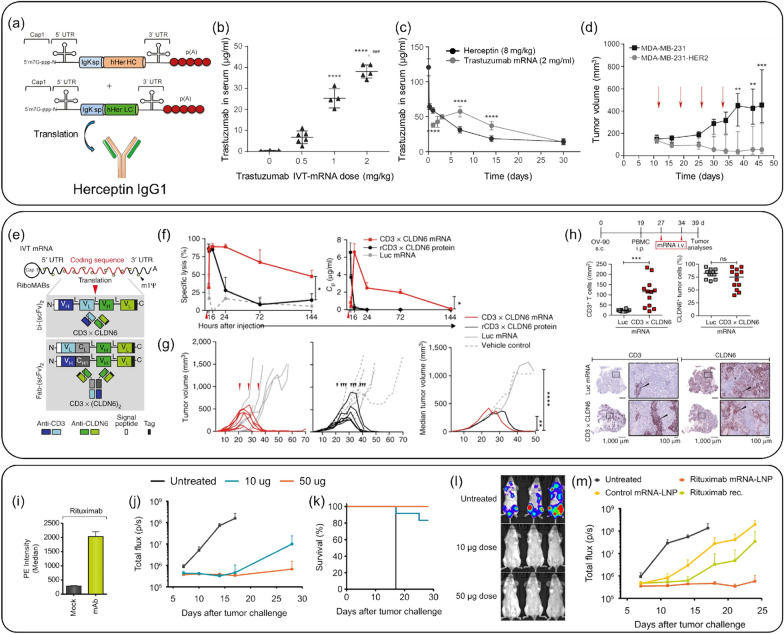
Fig. 8.
Antibody-encoded mRNA-LNP Delivery System. a Schematic representation of mRNAs encoding the heavy and light chains of trastuzumab. b Trastuzumab concentrations in C57BL/6 mouse serum 24 h after injection of cKK-E12 LNPs with trastuzumab mRNA via the tail vein at different doses. c Pharmacokinetics of trastuzumab in C57BL/6 mouse serum after a single i.v. dose of 8 mg/kg Herceptin (Genentech) or 2 mg/kg cKK-E12 LNPs with trastuzumab mRNA. d Growth of HER2-negative (MDA-MB-231) and HER2-positive (MDA-MB-231-HER2) tumors in mice treated with trastuzumab mRNA. Arrows indicate the days of mRNA-LNP injections. a–d: Reproduced from a previous report [8] with permission from Elsevier). e Structures of the IVT bi-(scFv)2 and Fab-(scFv)2 RiboMABs. f Ex vivo cytotoxicity (left) and concentration (Cp) (right) of endogenously translated CD3 × CLDN6 RiboMAB in the plasma of NSG mice after i.v. administration of polymer/lipid-formulated mRNA. g Mice were treated with CD3 × CLDN6 or luciferase mRNA (n = 6/group; three doses of 3 µg/mouse i.v. weekly) or with purified CD3 × CLDN6 protein (200 µg/kg) or vehicle (n = 7/group; three doses intraperitoneally (i.p.) weekly, total of ten doses). Tumor growth for individual mice (left, mRNA; right, recombinant protein) are shown. h Mice were treated with two doses of CD3 × CLDN6 mRNA (n = 4) or luciferase mRNA as a negative control (n = 4) (both 3 µg/mouse i.v. weekly). Tumor-infiltrating lymphocytes (human CD3 + cells; left) and CLDN6-expressing tumor cells (right) were quantified by immunohistochemistry in three consecutive tumor sections. e–h: Reproduced from a previous report [121] with permission from Springer Nature). i Binding of mRNA-encoded rituximab expressed in BHK cells to Raji cells. Depicted is the median of phycoerythrin (PE) fluorescence of all living cells. j–m mRNA-encoded mAb protects mice from lethal tumor challenge. Each group comprised 12 mice. j Tumor development assessed by whole-body luminescence imaging at indicated times after tumor challenge. k Survival of mice receiving i.v. injections of either 10 or 50 µg of mRNA-LNP encoding rituximab. l Representative luminescence images of mice treated with two different doses of mRNA-LNP encoding rituximab or untreated mice at day 13 after tumor challenge. m Tumor development of mice receiving i.v. injections of 50 µg of mRNA-LNP encoding rituximab or control antibody or 200 µg of recombinant rituximab. The experiment was assessed by whole-body luminescence imaging at indicated times after tumor challenge.
(Reproduced from a previous report [122] with permission from EMBO Press)