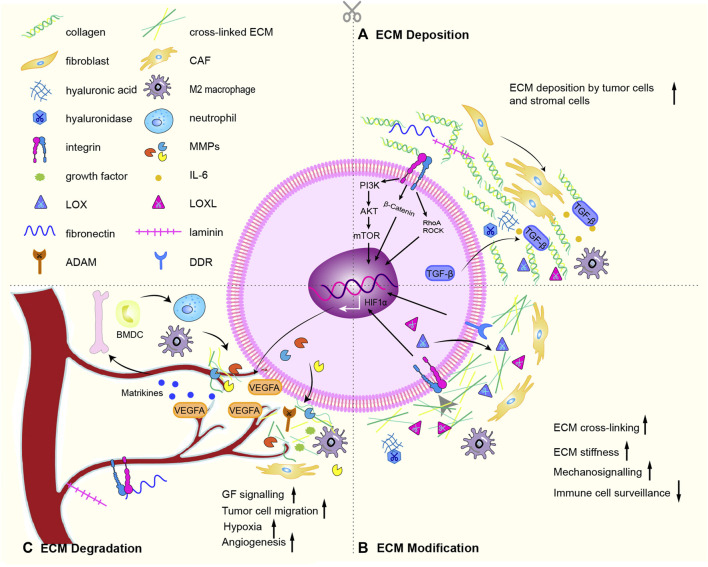
FIGURE 2.
ECM remodeling mechanism. (A) Especially through active TGF-β signaling, CAFs deposit abundant collagen and express cytokines, promotes the recruitment and activation of M2 macrophages. The deposition of cells inevitably lead to deposition of ECM subassembly, including collagen, ECM modifying enzymes (LOX, LOX-like protein), fibronectin, hyaluronic acid, tenascin C and laminin. (B) Chemical modification alters the biochemical properties and structural characteristics of the ECM. The main proteins involved are LOX, LOXLs, and TG2. Force-mediated ECM remodeling affects ECM tissue by aligning ECM fibers and opening cell migration channels. The main mechanical sensors involved are integrins, ROCK, DDR, Piezo1, YAP/TAZ. Overexpression of LOX and LOXLs increases fibrosis and ECM stiffness, and promotes tumorigenesis and metastasis. During this modification, mechanical forces exerted by aggregated integrins would result in nonproteolytic destruction of the basement membrane, allowing invasion of cancer cells. (C) CAFs, cancer cells and recruited BMDC secrete proteases that degrade the ECM: such as MMPs, disintegrins and ADAMs. ECM degradation is an important driver of cancer cell motility. The binding of soluble signaling molecules such as growth factors to the ECM renders them insoluble and inactive, while proteases enables their release. Proteolytic ECM degradation produces bioactive matrikines and releases matrix-bound matrikines. These factors induce pro-tumor ECM signaling that promotes tumor proliferation, migration, invasion, and angiogenesis. Matrikines also induce the activation of BMDC to secrete neutrophils. Neutrophils secrete potent MMP-9, which degrades ECM and releases matrix-bound VEGF, creating a concentration gradient of new angiogenic sprouts. Finally, upon stimulation of dense ECM, tumor cells may acquire endothelial-like functions and mimic the vasculature connected to blood vessels. Some graphic elements refer to published literature (Winkler et al., 2020).