Abstract
The enantioselective reaction of 1,6-enynes with O-, N-, and C-nucleophiles has been developed by matched ion pair gold(I) catalysis in which the chiral gold(I) cation and anion are H-bonded through a urea group. Very high levels of enantiocontrol are achieved (up to >99:1 er) for a broad scope of substrates. DFT studies demonstrate the importance of the H-bond donor group in anchoring the matched chiral cation- and anion-favoring additional noncovalent interactions.
Keywords: hydrogen bond interaction, chiral counterion, gold(I) catalysis, urea, phosphoramide, match−mismatch
Although the field of gold(I) catalysis has experienced an exponential growth in past decades,1−9 the development of enantioselective transformations with broader scope has been more difficult.10−13 After the pioneering work by Toste et al. on gold asymmetric counterion-directed catalysis (ACDC) for the enantioselective cyclization of allenes using chiral phosphate salts in combination with achiral digold complexes (Figure 1),14−17 the use of that concept has been used for the activation of alkyne18−22 or allene-containing substrates23−27 to circumvent some of the limitations in enantioselective gold(I) catalysis.28−30
Figure 1.
Gold(I) in the ACDC and HCDC strategies.
Our group recently introduced H-bonded counterion-directed catalysis (HCDC) (Figure 1) using achiral gold catalysts containing urea or squaramide H-bond donor motifs.31,32 In this approach, the H-bond donor facilitates the ligand-substrate exchange step and fixes the chiral information close to the reaction center, thereby allowing for an efficient transfer of the stereochemical information in cyclization reactions.33−36
Herein, we present the application of the HCDC approach with chiral binol-based phosphite gold(I) complexes37 equipped with urea groups together with matched chiral counterions for the enantioselective gold(I)-catalyzed nucleophilic addition to 1,6-enynes, which takes place with excellent enantioselectivities.
A library of phosphite gold(I) complexes carrying a urea was easily prepared in a one-pot, two-step procedure starting from chiral (R)-binaphthols with the desired 3,3′-substitution pattern or from achiral resorcinol [4]arenes (Figure 2). Additionally, chiral silver salts were prepared from commercially available chiral BINOLs and related biphenols (Figure 2). Urea groups in the para-, meta-, or ortho-position with respect to the phosphite were introduced to test the directing effect of the H-bond donor. We envisioned that chiral phosphoramidate silver salts would form more reactive catalysts since they are less basic than their corresponding phosphoric acid counterparts and, therefore, more easily substituted by the unsaturated substrates from the gold(I) coordination sphere.31
Figure 2.

Library of chiral gold(I) complexes and Ag(I) salts. aCYLview representations are for X-ray crystallography structures of Au4 and Au11. Solvent molecules are omitted for clarity. Au4 forms dimers in the solid state. Color code: P, orange; Au, yellow; Cl, green; O, red; N, blue; C, gray; and H, white. anthr = anthracenyl, phenant = phenantracenyl.
Our system proved to be particularly efficient in the addition of nucleophiles to 1,6-enynes.38−52 We first examined, using high-throughput experimentation (HTE), the addition of indole to 1a(40,41) with different chiral gold(I) complexes and silver(I) salts.53 The best combinations were then scaled up to 0.05 mmol (Table 1). Gold(I) complexes with the urea in the para-position showed much higher reactivity than those with ureas at ortho or meta. Substituents in the 3,3′-position of the BINOL scaffolds in both the Au(I) catalyst and Ag salt also had an important impact. Through the use of toluene as solvent, a clear match-missmatch scenario was observed using (R)-Au4 with the (R)- or (S)-enantiomers of Ag6, which led to 1a with 95:5 er and 61.5:38.5 er, respectively (Table 1, entries 4 and 7). This observation was also present in other examples. For example, EtOH was used as a nucleophile with (R)-Au1 or (R)-Au4 in combination with the (R)- or (S)-enantiomers of the same Ag salt (Table 1, entries 8–11). Better yields and enantioselectivities were obtained in 1,4-dioxane. Thus, the combination of (R)-Au4 with the (R)-enantiomers of Ag1, Ag6, or Ag8 provided 1a in >99:1, 97.5:2.5, and 95:5 er, respectively (Table 1, entries 13–15). Control experiments showed that neither (R)-Au4 nor (R)-Ag1 was active on its own (Table 1, entries 17 and 18). Whereas cavitand Au9,52 equipped with para-urea groups, gives satisfactory results with (R)-Ag6 (Table 1, entry 16), achiral Au(I) complex Au12, which was found to be the optimal for the intramolecular formal [4 + 2] cycloaddition of arylalkynes with alkenes,31 together with (R)-Ag1, showed poor reactivity and low enantioinduction in the formation of 2a (Table 1, entry 19). Silver salt AgSbF6 in combination with (R)-Au4 (Table 1, entry 20) gave lower yields and enantioselectivities than those observed with the dual-matched chiral system.53
Table 1. Enantioselective Gold(I)-Catalyzed Addition of Indole to 1,6-Enyne 1aa.
| entry | [Au] | [Ag] | NuH | time (h) | yield (%)b | erc |
|---|---|---|---|---|---|---|
| 1 | Au1 | (R)-Ag6 | Indd | 44 | 61 | 78:22 |
| 2 | Au2 | (R)-Ag6 | Indd | 44 | 69 | 54:46 |
| 3 | Au3 | (R)-Ag6 | Indd | 44 | 6 | 50:50 |
| 4 | Au4 | (R)-Ag6 | Indd | 44 | 82 | 95:5 |
| 5 | Au5 | (R)-Ag6 | Indd | 44 | 74 | 60:40 |
| 6 | Au6 | (R)-Ag6 | Indd | 44 | 39 | 54:46 |
| 7 | Au4 | (S)-Ag6 | Indd | 44 | 61 | 61.5:38.5 |
| 8 | Au4 | (R)-Ag6 | EtOHd | 14 | 87 | 98:2 |
| 9 | Au4 | (S)-Ag6 | EtOHd | 14 | 85 | 57:43 |
| 10 | Au1 | (R)-Ag6 | EtOHd | 14 | 67 | 91:9 |
| 11 | Au1 | (S)-Ag6 | EtOHd | 14 | 53 | 47:53 |
| 12 | Au1 | (R)-Ag1 | Inde | 14 | 82 | 91:9 |
| 13 | Au4 | (R)-Ag1 | Inde | 14 | 93 | >99:1 |
| 14 | Au4 | (R)-Ag6 | Inde | 14 | 98 | 97.5:2.5 |
| 15 | Au4 | (R)-Ag8 | Inde | 14 | 90 | 95:5 |
| 16 | Au9 | (R)-Ag6 | Inde | 18 | 78 | 94:6 |
| 17 | Au4 | Inde | 24 | 0 | ||
| 18 | (R)-Ag1 | Inde | 24 | 0 | ||
| 19 | Au12 | (R)-Ag1 | Inde | 24 | 18 | 52:48 |
| 20 | Au4 | AgSbF6 | Inde | 24 | 76 | 92.5:7.5 |
Reactions carried out under Ar or N2 at a 0.05 mmol scale, at 27 °C with (R)-configured Au(I) complexes.
Yields determined by 1H NMR using dodecane as internal standard.
er determined by supercritical fluid chromatography (SFC) using a chiral stationary phase.
Toluene (0.1 M) was used as solvent.
1,4-dioxane (0.1 M) was used as solvent. NuH = nucleophile. Ind = Indole.
It is interesting that, whereas in our system the matched pair is achieved with (R)-Au cation and (R)-phosphoramidate anion, in systems based on two BINOL-based cation/anion ion pairs, the matched catalyst resulted from the (R)/(S) ion combination.17,24,26
We examined the scope of the reaction using the optimal combination (R)-Au4 and (R)-Ag1 (Scheme 1). The reactions of 1,6-enyne 1a were performed at 2 mol% catalyst loading in a 0.100 mmol scale, and two conditions were used depending on the nature of the nucleophile and the reactivity of the enyne: conditions A using 1,4-dioxane at 27 °C or conditions B using toluene at 27, 0, or −10 °C. Substituents in the 1, 2, and 5-positions of indole were well tolerated to give adducts 2b–e. Electron-rich arenes, such as 1,3,5-trimethoxybenzne, 1,3-dimethoxybenzene, and N,N-dimethylaniline, led to 2f–h in excellent yields and enantioselectivities. Similarly, excellent results were also obtained with heteroatom-centered nucleophiles, such as anilines; a carbamate; alcohols; and water to give 2i–r in excellent yields and enantioselectivities.
Scheme 1. Enantioselective Addition of O-, N-, and C-Nucleophiles to 1,6-Enynes.
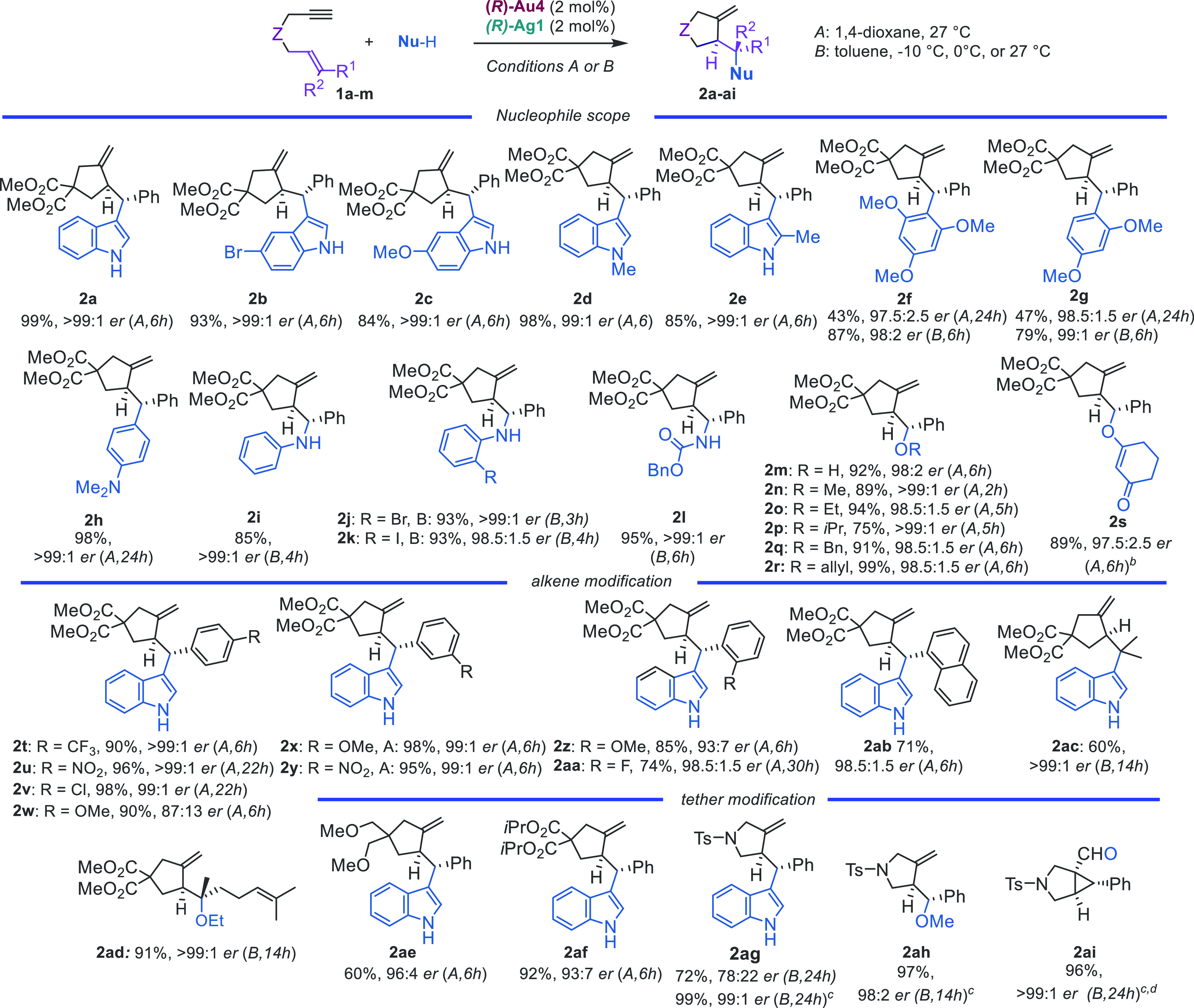
Reaction performed under Ar or N2 in anhydrous solvent (0.1 M). Yields given for isolated material after purification; er values were determined by HPLC or SFC on chiral stationary phase. Products were obtained as single diastereomers. Reaction times are in parentheses.
1,3-Cyclohexandione (2.0 equiv) was used as nucleophile.
Reaction carried out using (R)-Au7 and (R)-Ag5 at a 3 mol% catalyst loading.
Diphenylsulfoxide (1.5 equiv) was used as nucleophile.
1,6-Enynes with different substitutions at the alkene also gave the corresponding adducts 2t–ad in good to excellent yields and high enantioselectivities, except for those with a para- or ortho-anisyl group, which gave 2w and 2z in 87:13 and 93:7 er (Scheme 1). The absolute configurations of 2v and 2ai were determined to be the (S,S) and (R,S) by X-ray diffraction.54 Changing the malonate to a dimethyl ether tether favored the formation of the cycloisomerization product, thereby leading to 2ae in moderate yield. However, increasing the size of the ester from methyl to isopropyl reduced the enantioselectivity to a 93:7 er in 2af, which suggests that the tether plays an important role in the folding of the 1,6-enyne in the chiral pocket of the catalyst.
Changing the malonate tether in the 1,6-enyne to a N-tosyl led to a decrease in the enantioselectivity, which provided 2ag in 78:22 er. (Scheme 1) However, using (R)-Au7 together with (R)-Ag5 gave adducts 2ag and 2ah, as well as aldehyde 2ai, which resulted from the oxidation of the gold(I) carbene intermediate with diphenylsulfoxide,55 in 98:2 to >99:1 er.
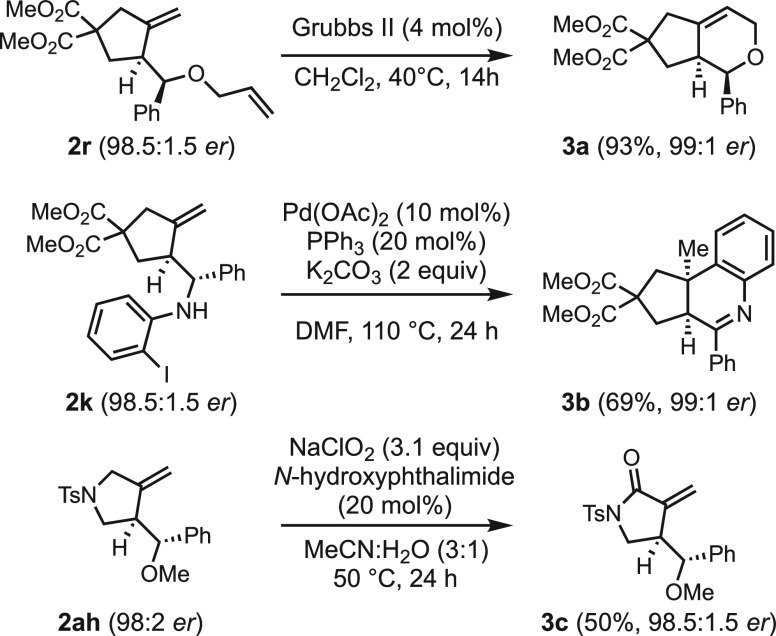
To further demonstrate the utility of this enantioselective addition of nucleophiles to 1,6-enynes, selected transformations into more complex products were performed (Scheme 2). Thus, the ring-closing metathesis of 2r with the second generation Grubbs catalyst56 gave bicyclic derivative 3a with no erosion on the enantioselectivity. The ortho-iodoaniline addition product 2k underwent an intramolecular Heck reaction with the concomitant formation of a new stereocenter to afford 2,3,3a,9b-tetrahydro-1H-cyclopenta[c]quinoline 3b.50 Finally, the allylic position in pyrrolidine 2ah was oxidized with NaClO2 and N-hydroxyphthalimide to give lactam 3c with 98.5:1.5 er.
Scheme 2. Product Derivatization.
DFT studies were conducted [B3LYP-D3/6-31G(d) (C, H, P, O, F, N, S)//B3LYP-D3/6-311G(d,p) and SDD (Au), PCM = toluene]57 to gain insight into the role of the urea and the possible secondary interactions involved in the enantioselective cyclization step using chiral (R)-Au4 and chiral counterion from (R)-Ag6, which provided 2a in 95:5 er.
Our computations predicted a Curtin–Hammett scenario (Scheme 3a), where the two orientations of the alkenes (int1a and int3a) are in equilibrium. Although int3a is 4.9 kcal/mol more stable than int1a, the major product arises from the latter via TSint1a-2a, which is lower in energy than TSint3a-4a, thereby giving rise to product 2a with an S configuration by reaction through the Re face of the alkene, which agrees with the experimental results.
Scheme 3. DFT Calculations for the Enantiodetermining Step.
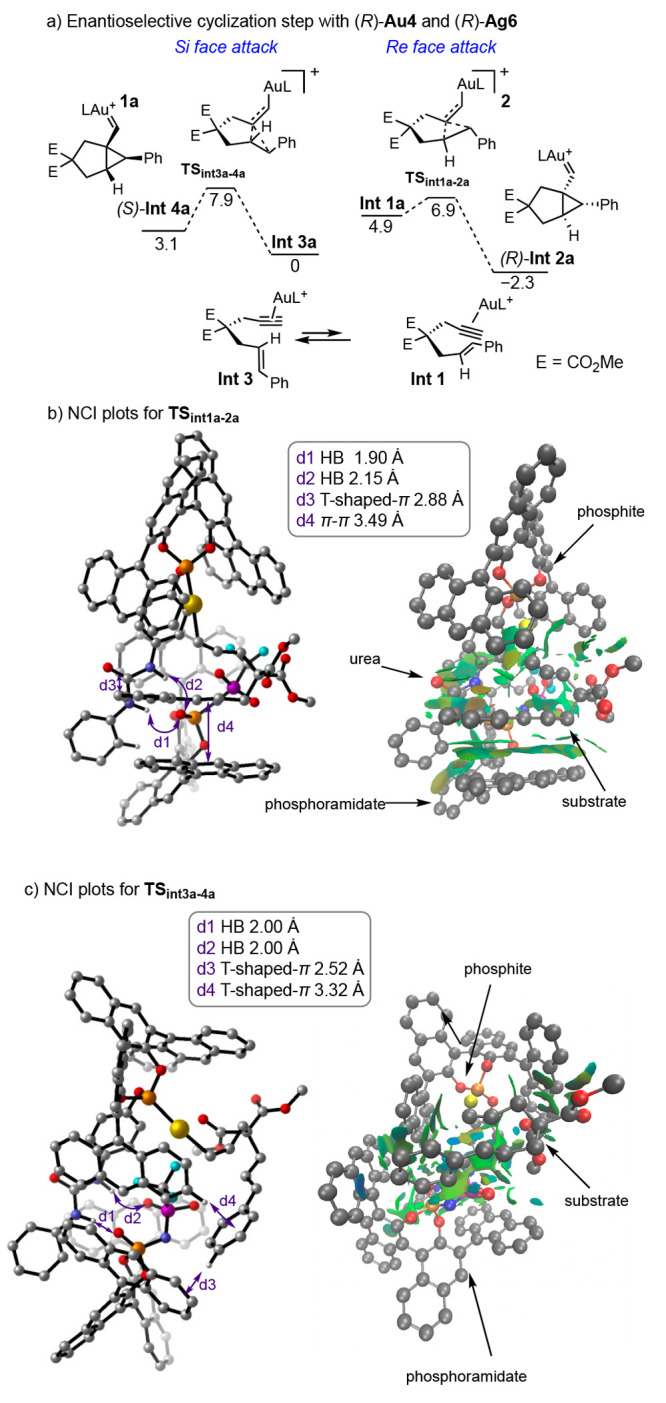
Two most relevant pathways for the enantiodetermining cyclization step of 1a with (R)-Au4 and (R)-Ag6. NCI plots and CYLview representations for TSint1a-2a and TSint3a-4a. Hydrogens are omitted for clarity, except relevant ones. Strong attractive interactions are blue, weak attractive interactions are green, and strong repulsive interactions are red. Color code: P, orange; Au, yellow; F, cyan; O, red; N, blue; S, purple; C, gray; and H, white. Energy values are in kcal/mol. HB = hydrogen bond.
Noncovalent interaction (NCI) plots were performed to visualize the noncovalent interactions in the two possible transition states (Scheme 3b,c) which revealed that the H-bond interactions from the urea and phosphoramidate group act as anchors of the two parts and favor the additional interactions that stabilize the transition state TSint1a-2a. Apart from the strong H-bonding interactions, T-shaped-π interactions between the ortho-C–H of the N-phenyl urea and the π-system of the BINOL counterion were observed. A strong extended attractive sandwich π–π interaction between the π-system of the cinnamyl alkene of the substrate and one anthracenyl group of the chiral counterion were observed in TSint1a-2a. However, for TSint3a-4a, a T-shaped π-attractive interaction was found between the C–H in para position to the alkene in the aryl ring of the substrate and the anthracene of the counterion, which helped to stabilize the transition state.
Finally, other reactions were tested by combining BINOL-derived gold(I) catalysts and different silver(I) salts as chloride scavengers, but the optimal ion pair combination could not be found. We discovered that 1,6-enynes bearing internal aryl-substituted alkynes led to formal products of [4 + 2] cycloaddition31 with poor yields and moderate enantioselectivities, most likely because of the small pocket generated by the ion pair. However, when using chiral gold(I) complexes and small achiral counterions, such as AgSbF6, promising enantioselectivities were found (95:5 er). We also tested the [2 + 2] cycloaddition of phenylacetylene with alkenes,58 but no promising combination was observed.
In summary, we have developed the enantioselective nucleophilic addition of hetero- and carbonucleophiles that proceeds with the broadest scope and highest enantioselectivity using a chiral catalyst with a chirally matched gold(I) phosphitourea and phosphoramidate, which can be readily prepared from commercially available BINOLs. A model for the enantioinduction has been proposed on the basis of DFT calculations and NCI plots where the urea in the chiral gold(I) cation anchors the chiral counterion in close proximity creating a chiral pocket to fold the unsaturated substrate.
Acknowledgments
We thank the MCIN/AEI/10.13039/501100011033 (PID2019-104815GB-I00 and CEX2019-000925-S), the MUniv (FPU20/0255 Fellowship to À.M.), the European Research Council (Advanced Grant 835080), the AGAUR (2021 SGR 01256), and CERCA Program/Generalitat de Catalunya for financial support. We also thank Dr. Imma Escofet for assistance with the computational calculations, as well as the ICIQ X-ray diffraction, NMR, and the chromatography and mass spectrometry units.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.3c02638.
Optimization tables, procedures, characterization, NMR spectra, SFC and HPLC traces, DFT computations, and crystallographic data (PDF)
Accession Codes
CCDC 2266405–2266408 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: + 44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- Hashmi A. S. K. Homogeneous gold catalysts and alkynes: A successful liaison. Gold Bull. 2003, 36, 3–9. 10.1007/BF03214859. [DOI] [Google Scholar]
- Zhang L.; Sun J.; Kozmin S. A. Gold and platinum catalysis of enyne cycloisomerization. Adv. Synth. Catal. 2006, 348, 2271–2296. 10.1002/adsc.200600368. [DOI] [Google Scholar]
- Fürstner A.; Davies P. W. Catalytic Carbophilic Activation: Catalysis by Platinum and Gold π Acids. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. 10.1002/anie.200604335. [DOI] [PubMed] [Google Scholar]
- Fensterbank L.; Malacria M. Molecular complexity from polyunsaturated substrates: the gold catalysis approach. Acc. Chem. Res. 2014, 47, 953–965. 10.1021/ar4002334. [DOI] [PubMed] [Google Scholar]
- Dorel R.; Echavarren A. M. Gold (I)-catalyzed activation of alkynes for the construction of molecular complexity. Chem. Rev. 2015, 115, 9028–9072. 10.1021/cr500691k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiri A. M.; Hashmi A. S. K. Gold-catalysed reactions of diynes. Chem. Soc. Rev. 2016, 45, 4471–4503. 10.1039/C6CS00023A. [DOI] [PubMed] [Google Scholar]
- Boyle J. W.; Zhao Y.; Chan P. W. H. Product Divergence in Coinage-Metal-Catalyzed Reactions of π-Rich Compounds. Synth. 2018, 50, 1402–1416. 10.1055/s-0036-1591762. [DOI] [Google Scholar]
- Mato M.; Franchino A.; García-Morales C.; Echavarren A. M. Gold-catalyzed synthesis of small rings. Chem. Rev. 2021, 121, 8613–8684. 10.1021/acs.chemrev.0c00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau D.; Rayo D. F. L.; Mansour A.; Muratov K.; Gagosz F. Gold-catalyzed reactions of specially activated alkynes, allenes, and alkenes. Chem. Rev. 2021, 121, 8756–8867. 10.1021/acs.chemrev.0c00788. [DOI] [PubMed] [Google Scholar]
- Milcendeau P.; Sabat N.; Ferry A.; Guinchard X. Gold-catalyzed enantioselective functionalization of indoles. Org. Biomol. Chem. 2020, 18, 6006–6017. 10.1039/D0OB01245A. [DOI] [PubMed] [Google Scholar]
- Jiang J. J.; Wong M. K. Recent advances in the development of chiral gold complexes for catalytic asymmetric catalysis. Chem.—Asian J. 2021, 16, 364–377. 10.1002/asia.202001375. [DOI] [PubMed] [Google Scholar]
- Zuccarello G.; Escofet I.; Caniparoli U.; Echavarren A. M. New-Generation Ligand Design for the Gold-Catalyzed Asymmetric Activation of Alkynes. ChemPlusChem. 2021, 86, 1283–1296. 10.1002/cplu.202100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escofet I.; Zuccarello G.; Echavarren A. M. Gold-catalyzed enantioselective cyclizations and cycloadditions. Adv. Organomet. Chem. 2022, 77, 1–42. 10.1016/bs.adomc.2022.01.003. [DOI] [Google Scholar]
- Hamilton G. L.; Kang E. J.; Mba M.; Toste F. D. A powerful chiral counterion strategy for asymmetric transition metal catalysis. Science 2007, 317, 496–499. 10.1126/science.1145229. [DOI] [PubMed] [Google Scholar]
- LaLonde R. L.; Wang Z. L.; Mba M.; Lackner A. D.; Toste F. D. Gold(I)-Catalyzed Enantioselective Synthesis of Pyrazolidines, Isoxazolidines, and Tetrahydrooxazines. Angew. Chem., Int. Ed. 2010, 49, 598–601. 10.1002/anie.200905000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad A. K.; Leutzow J.; Czekelius C. Anion-Induced Enantioselective Cyclization of Diynamides to Pyrrolidines Catalyzed by Cationic Gold Complexes. Angew. Chem., Int. Ed. 2012, 51, 11149–11152. 10.1002/anie.201205416. [DOI] [PubMed] [Google Scholar]
- Miles D. H.; Veguillas M. F.; Toste F. D. Gold(i)-catalyzed enantioselective bromocyclization reactions of allenes. Chem. Sci. 2013, 4, 3427–3431. 10.1039/c3sc50811k. [DOI] [Google Scholar]
- Spittler M.; Lutsenko K.; Czekelius C. Total synthesis of (+)-mesembrine applying asymmetric gold catalysis. J. Org. Chem. 2016, 81, 6100–6105. 10.1021/acs.joc.6b00985. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Smal V.; Retailleau P.; Voituriez A.; Frison G.; Marinetti A.; Guinchard X. Tethered counterion-directed catalysis: merging the chiral ion-pairing and bifunctional ligand strategies in enantioselective gold(I) catalysis. J. Am. Chem. Soc. 2020, 142, 3797–3805. 10.1021/jacs.9b11154. [DOI] [PubMed] [Google Scholar]
- Pedrazzani R.; An J.; Monari M.; Bandini M. New Chiral BINOL-Based Phosphates for Enantioselective [Au(I)]-Catalyzed Dearomatization of β-Naphthols with Allenamides. Eur. J. Org. Chem. 2021, 2021, 1732–1736. 10.1002/ejoc.202100166. [DOI] [Google Scholar]
- Yu Y.; Zhang Z.; Voituriez A.; Rabasso N.; Frison G.; Marinetti A.; Guinchard X. Enantioselective Au(I)-catalyzed dearomatization of 1-naphthols with allenamides through Tethered Counterion-Directed Catalysis. Chem. Commun. 2021, 57, 10779–10782. 10.1039/D1CC04088J. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Sabat N.; Frison G.; Marinetti A.; Guinchard X. Enantioselective Au(I)-Catalyzed Multicomponent Annulations via Tethered Counterion-Directed Catalysis. ACS Catal. 2022, 12, 4046–4053. 10.1021/acscatal.2c00120. [DOI] [Google Scholar]
- Aikawa K.; Kojima M.; Mikami K. Axial chirality control of gold (biphep) complexes by chiral anions: application to asymmetric catalysis. Angew. Chem., Int. Ed. 2009, 48, 6073–6077. 10.1002/anie.200902084. [DOI] [PubMed] [Google Scholar]
- Aikawa K.; Kojima M.; Mikami K. Synergistic Effect: Hydroalkoxylation of Allenes through Combination of Enantiopure BIPHEP-Gold Complexes and Chiral Anions. Adv. Synth. Catal. 2010, 352, 3131–3135. 10.1002/adsc.201000672. [DOI] [Google Scholar]; Aikawa K.; Kojima M.; Mikami K. Adv. Synth. Catal. 2011, 353, 2882–2883. 10.1002/adsc.201100838. [DOI] [Google Scholar]
- Barreiro E. M.; Broggini D. F. D.; Adrio L. A.; White A. J. P.; Schwenk R.; Togni A.; Hii K. K. Gold (I) complexes of conformationally constricted chiral ferrocenyl phosphines. Organometallics 2012, 31, 3745–3754. 10.1021/om300222k. [DOI] [Google Scholar]
- Handa S.; Lippincott D. J.; Aue D. H.; Lipshutz B. H. Asymmetric gold-catalyzed lactonizations in water at room temperature. Angew. Chem., Int. Ed. 2014, 53, 10658–10662. 10.1002/anie.201404729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi W.; Toste F. D. Gold (I)-Catalyzed Enantioselective Desymmetrization of 1, 3-Diols through Intramolecular Hydroalkoxylation of Allenes. Angew. Chem., Int. Ed. 2015, 54, 14447–14451. 10.1002/anie.201508331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh C.; Enders D. Merging organocatalysis and gold catalysis-a critical evaluation of the underlying concepts. Chem.—Eur. J. 2012, 18, 10212–10225. 10.1002/chem.201200287. [DOI] [PubMed] [Google Scholar]
- Inamdar S.; Konala A.; Patil N. T. When gold meets chiral Brønsted acid catalysts: extending the boundaries of enantioselective gold catalysis. Chem. Commun. 2014, 50, 15124–15135. 10.1039/C4CC04633A. [DOI] [PubMed] [Google Scholar]
- Bao M.; Zhou S.; Hu W.; Xu X. Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization. Chin. Chem. Lett. 2022, 33, 4969–4979. 10.1016/j.cclet.2022.04.050. [DOI] [Google Scholar]
- Franchino A.; Martí C0.; Echavarren A. M. H-Bonded Counterion-Directed Enantioselective Au(I) Catalysis. J. Am. Chem. Soc. 2022, 144, 3497–3509. 10.1021/jacs.1c11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí C0.; Montesinos-Magraner M.; Echavarren A. M.; Franchino A. H-Bonded Counterion-Directed Catalysis: Enantioselective Gold(I)-Catalyzed Addition to 2-Alkynyl Enones as a Case Study. Eur. J. Org. Chem. 2022, 2022 (38), e202200518 10.1002/ejoc.202200518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandee A. J.; van der Burg A. M.; Reek J. N. H. UREAphos: Supramolecular Bidentate Ligands for Asymmetric Hydrogenation. Chem. Commun. 2007, 864–866. 10.1039/B614571J. [DOI] [PubMed] [Google Scholar]
- Meeuwissen J.; Kuil M.; van der Burg A. M.; Sandee A. J.; Reek J. N. H. Application of a Supramolecular-Ligand Library for the Automated Search for Catalysts for the Asymmetric Hydrogenation of Industrially Relevant Substrates. Chem.—Eur. J. 2009, 15, 10272–10279. 10.1002/chem.200901110. [DOI] [PubMed] [Google Scholar]
- Meeuwissen J.; Reek J. N. H. Supramolecular Catalysis beyond Enzyme Mimics. Nat. Chem. 2010, 2, 615–621. 10.1038/nchem.744. [DOI] [PubMed] [Google Scholar]
- Reek J. N. H.; de Bruin B.; Pullen S.; Mooibroek T. J.; Kluwer A. M.; Caumes X. Transition Metal Catalysis Controlled by Hydrogen Bonding in the Second Coordination Sphere. Chem. Rev. 2022, 122, 12308–12369. 10.1021/acs.chemrev.1c00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpont N.; Escofet I.; Pérez-Galán P.; Spiegl D.; Raducan M.; Bour C.; Sinisi R.; Echavarren A. M. Modular chiral gold(i) phosphite complexes. Catal. Sci. Technol. 2013, 3, 3007–3012. 10.1039/c3cy00250k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; Muñoz M. P.; Buñuel E.; Nevado C.; Cárdenas D. J.; Echavarren A. M. Cationic Gold(I) Complexes: Highly Alkynophylic Catalysts for the Exo- and Endo-Cyclization of Enynes. Angew. Chem., Int. Ed. 2004, 43, 2402–2406. 10.1002/anie.200353207. [DOI] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; Muñoz M. P.; López S.; Jiménez-Núñez E.; Nevado C.; Herrero-Gómez E.; Raducan M.; Echavarren A. M. Gold(I)-Catalyzed Cyclizations of 1,6-Enynes: Alkoxycyclizations and Exo/Endo Skeletal Rearrangements. Chem.—Eur. J. 2006, 12, 1677–1693. 10.1002/chem.200501088. [DOI] [PubMed] [Google Scholar]
- Toullec P. Y.; Genin E.; Leseurre L.; Genet J.-P.; Michelet V. Room-Temperature AuI-Catalyzed C-C Bond Formation through a Tandem Friedel-Crafts-Type Addition/Carbocyclization Reaction. Angew. Chem., Int. Ed. 2006, 45, 7427–7430. 10.1002/anie.200601980. [DOI] [PubMed] [Google Scholar]
- Amijs C. H. M.; Ferrer C.; Echavarren A. M. Gold(I)-Catalysed Arylation of 1,6-Enynes: Different Site Reactivity of Cyclopropyl Gold Carbenes. Chem. Commun. 2007, 698–700. 10.1039/B615335F. [DOI] [PubMed] [Google Scholar]
- Amijs C. H. M.; López-Carrillo V.; Raducan M.; Pérez-Galán P.; Ferrer C.; Echavarren A. M. Gold(I)-Catalyzed Intermolecular Addition of Carbon Nucleophiles to 1,5- and 1,6-Enynes. J. Org. Chem. 2008, 73, 7721–7730. 10.1021/jo8014769. [DOI] [PubMed] [Google Scholar]
- Chao C. M.; Genin E.; Toullec P. Y.; Genêt J. P.; Michelet V. Towards Asymmetric Au-Catalyzed Hydroxy- and Alkoxycyclization of 1,6-Enynes. J. Organomet. Chem. 2009, 694, 538–545. 10.1016/j.jorganchem.2008.08.008. [DOI] [Google Scholar]
- Leseurre L.; Chao C. M.; Seki T.; Genin E.; Toullec P. Y.; Genêt J. P.; Michelet V. Synthesis of Functionalized Carbo- and Heterocycles via Gold-Catalyzed Cycloisomerization Reactions of Enynes. Tetrahedron 2009, 65, 1911–1918. 10.1016/j.tet.2008.11.105. [DOI] [Google Scholar]
- Matsumoto Y.; Selim K. B.; Nakanishi H.; Yamada K.; Yamamoto Y.; Tomioka K. Chiral Carbene Approach to Gold-Catalyzed Asymmetric Cyclization of 1,6-Enynes. Tetrahedron Lett. 2010, 51, 404–406. 10.1016/j.tetlet.2009.11.039. [DOI] [Google Scholar]
- Pradal A.; Chao C. M.; Vitale M. R.; Toullec P. Y.; Michelet V. Asymmetric Au-Catalyzed Domino Cyclization/nucleophile Addition Reactions of Enynes in the Presence of Water, Methanol and Electron-Rich Aromatic Derivatives. Tetrahedron 2011, 67, 4371–4377. 10.1016/j.tet.2011.03.071. [DOI] [Google Scholar]
- Wang W.; Yang J.; Wang F.; Shi M. Axially Chiral N-Heterocyclic Carbene gold(I) Complex Catalyzed Asymmetric Cycloisomerization of 1,6-Enynes. Organometallics 2011, 30, 3859–3869. 10.1021/om2004404. [DOI] [Google Scholar]
- Yamada K. I.; Matsumoto Y.; Selim K. B.; Yamamoto Y.; Tomioka K. Steric Tuning of C 2-Symmetric Chiral N-Heterocyclic Carbene in Gold-Catalyzed Asymmetric Cyclization of 1,6-Enynes. Tetrahedron 2012, 68, 4159–4165. 10.1016/j.tet.2012.03.107. [DOI] [Google Scholar]
- Gung B. W.; Holmes M. R.; Jones C. A.; Ma R.; Barnes C. L. Structure-enantioselectivity Correlation in NHC-Au(I) Catalysis for 1,6-Enynecyclizations. Tetrahedron Lett. 2016, 57, 3912–3915. 10.1016/j.tetlet.2016.07.046. [DOI] [Google Scholar]
- Miller R.; Carreras J.; Muratore M. E.; Gaydou M.; Camponovo F.; Echavarren A. M. Broad Scope Aminocyclization of Enynes with Cationic JohnPhos-Gold(I) Complex as the Catalyst. J. Org. Chem. 2016, 81, 1839–1849. 10.1021/acs.joc.5b02607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugny C.; Del Rio N.; Koohgard M.; Vanthuyne N.; Lesage D.; Bijouard K.; Zhang P.; Meijide Suárez J.; Roland S.; Derat E.; Bistri-Aslanoff O.; Sollogoub M.; Fensterbank L.; Mouriès-Mansuy V. β-Cyclodextrin-NHC-Gold(I) Complex (β-ICyD)AuCl: A Chiral Nanoreactor for Enantioselective and Substrate-Selective Alkoxycyclization Reactions. ACS Catal. 2020, 10, 5964–5972. 10.1021/acscatal.0c00127. [DOI] [Google Scholar]
- Martín-Torres I.; Ogalla G.; Yang J. M.; Rinaldi A.; Echavarren A. M. Enantioselective Alkoxycyclization of 1,6-Enynes with Gold(I)-Cavitands: Total Synthesis of Mafaicheenamine C. Angew. Chemie. Int. Ed. 2021, 60, 9339–9344. 10.1002/anie.202017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See the Supporting Information for more details.
- a Comparison of the reported optical rotations of 2a–2d43 with those of our products on the basis of the absolute configurations of 2v and 2ai determined by X-ray diffraction shows that the originally assigned configuration in ref (43) should be reversed.53; b Similarly, the absolute configuration of two of the products (5a,b) reported in ref (52) should be reversed.
- Witham C. A.; Mauleon P.; Shapiro N. D.; Sherry B. D.; Toste F. D. Gold (I)-catalyzed oxidative rearrangements. J. Am. Chem. Soc. 2007, 129, 5838–5839. 10.1021/ja071231+. [DOI] [PubMed] [Google Scholar]
- Chao C. M.; Toullec P. Y.; Michelet V. Synthesis of polycyclic heterocycles via sequential Au-catalyzed cycloisomerization and Ru-catalyzed metathesis reactions. Tetrahedron Lett. 2009, 50, 3719–3722. 10.1016/j.tetlet.2009.04.001. [DOI] [Google Scholar]
- See the Supporting Information for details. A data set collection of computational results is available in the ioChem-BD repository and can be accessed https://iochem-bd.iciq.es/browse/handle/100/60425.; Álvarez-Moreno M.; De Graaf C.; Lopez N.; Maseras F.; Poblet J. M.; Bo C. Managing the Computational Chemistry Big Data Problem: The ioChem-BD Platform. J. Chem. Inf. Model. 2015, 55, 95–103. 10.1021/ci500593j. [DOI] [PubMed] [Google Scholar]
- García-Morales C.; Ranieri B.; Escofet I.; López-Suarez L.; Obradors C.; Konovalov A. I.; Echavarren A. M. Enantioselective Synthesis of Cyclobutenes by Intermolecular [2 + 2] Cycloaddition with Non-C2 Symmetric Digold Catalysts. J. Am. Chem. Soc. 2017, 139, 13628–13631. 10.1021/jacs.7b07651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.