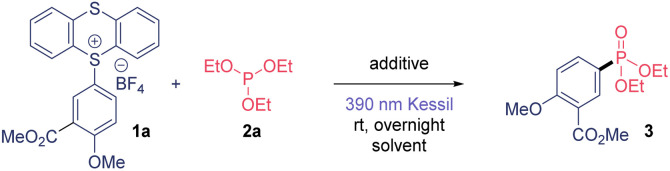
Optimization of the reaction conditionsa.
| |||||
|---|---|---|---|---|---|
| Entry | Equiv. 2a | Solvent | Additive | Light | Yieldb (%) |
| 1 | 10 | DMA | None | 390 nm | 65 |
| 2 | 10 | DMA | K2CO3 | 390 nm | 82 (75)c |
| 3 | 10 | DMA | DABCO | 390 nm | 80 |
| 4 | 10 | Acetone | K2CO3 | 390 nm | 81 |
| 5 | 10 | MeCN | K2CO3 | 390 nm | 88 |
| 6 | 10 | DCM | K2CO3 | 390 nm | 85 |
| 7 | 10 | MeCN | K2CO3 | 390 nm | 87 |
| 8 | 10 | MeCN | K3PO4 | 390 nm | 84 |
| 9 | 10 | MeCN | KHCO3 | 390 nm | 90 |
| 10 | 10 | MeCN | KHCO3 | Dark | <10 |
| 11 | 5 | MeCN | KHCO3 | 390 nm | 89 |
| 12 | 2.5 | MeCN | KHCO3 | 390 nm | 75 |
| 13 | 5 | MeCN | KHCO 3 | 390 nm | 87 d |
| 14 | 5 | MeCN | KHCO3 | 456 nm | 88 |
| 15 | 5 | Wet MeCN | KHCO3 | 390 nm | 53d |
Reaction conditions: 0.1 mmol of TT salt 1a, 0.1 mmol of the corresponding additive, indicated amounts of phosphite 2a and 0.1 M in the indicated dry solvent.
Determined by 1H NMR using 1,3,5-trimethoxybenzene as internal standard.
Isolated yield from 0.5 mmol of 1a.
30 minutes reaction time.
