Figure 1.
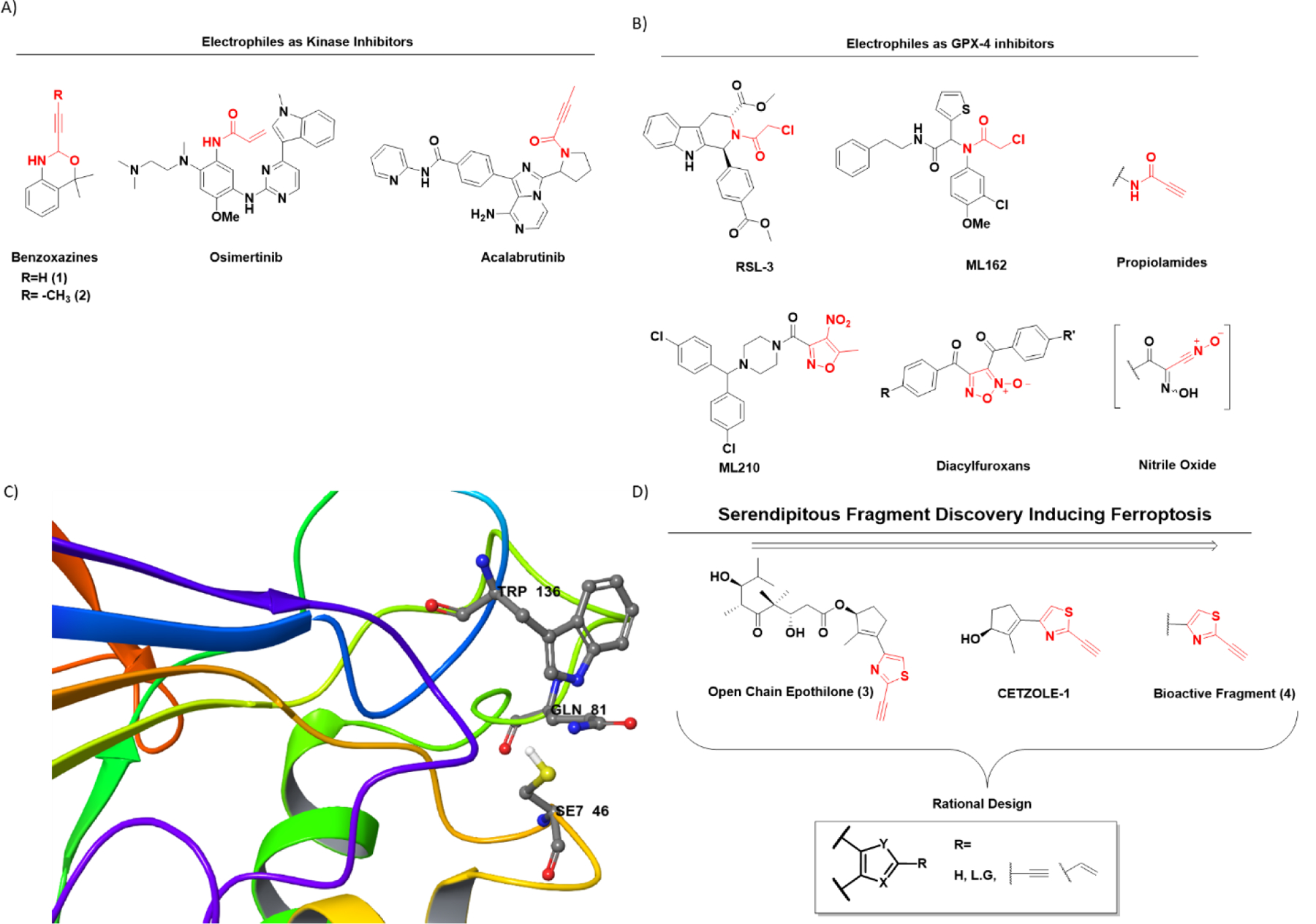
The heterocyclic warhead toolbox available for the design of covalent inhibitors. (A) Structures of some representative covalent kinase inhibitors. Typically, simple acrylamides have been used as electrophilic warheads of these molecules. Recently, McAulay et al.16 reported alkynyl benzoxazines (compounds 1 and 2) as potential kinase inhibitors. (B) Structures of several classical GPX4 inhibitors. Prototypical examples of covalent GPX4 inhibitors include RSL3 and ML162, both which have chloro-acetamide (C.A) as electrophilic warheads. New examples include ML210 and diacylfuroxans, which act as masked nitrile oxides (C) GPX4 active site representing the catalytic triad Se7 46, Gln81, and Trp136. The 3D model is generated using Maestro version 13.1 and protein data bank (PDB) entry with PDBid: 6ELW. The SeO2 residue in the original entry has been mutated in silico to SeH for more accurate representation. (D) Serendipitous discovery of thiazole-based electrophilic warheads following efforts to synthesize open-chain epothilones led to CETZOLE series of molecules. SAR studies reveal the thiazole fragment as the bioactive moiety.
