Scheme 3.
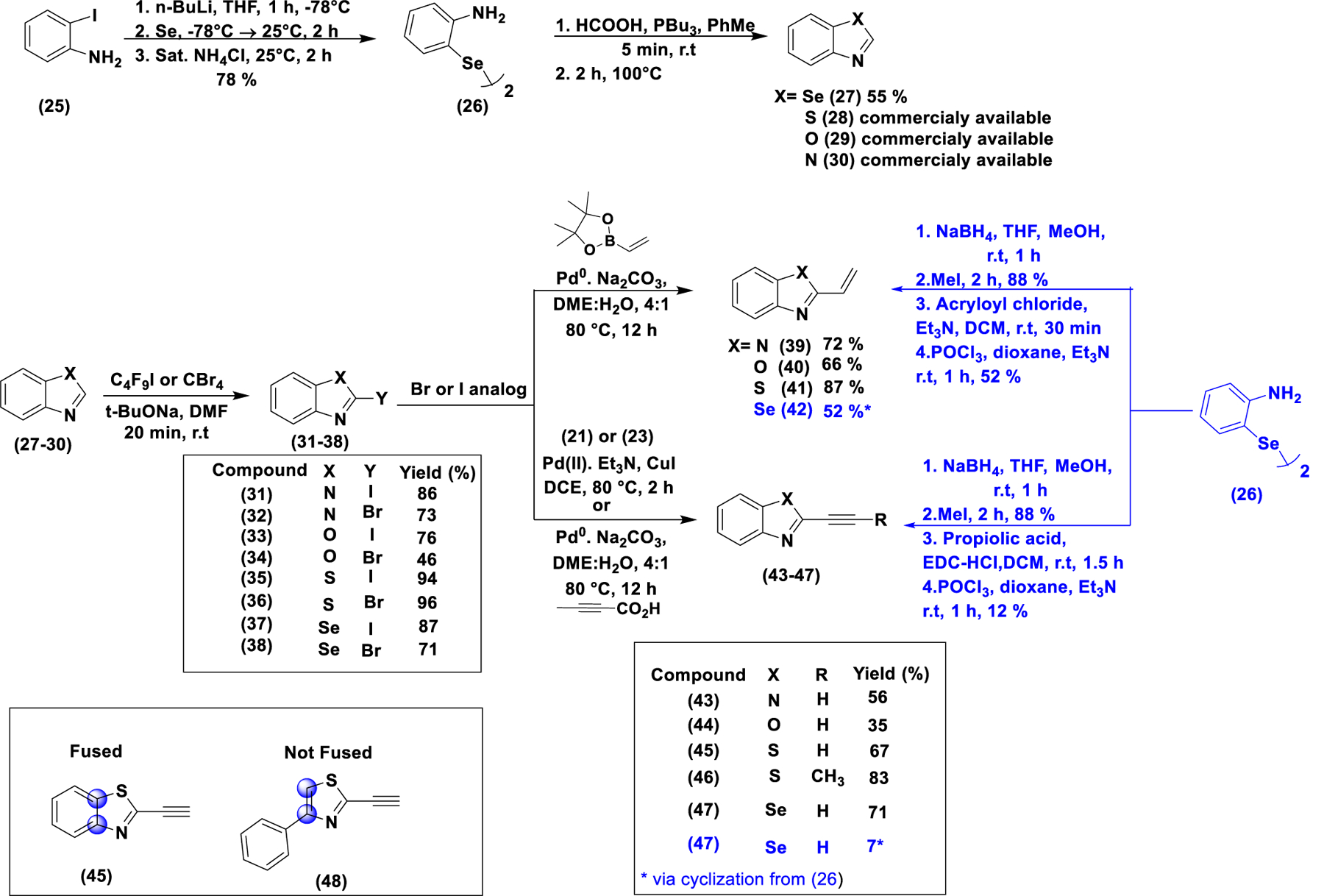
Synthesis of Benzo[d]heterocylces differently substituted at the 2-position with H, Br, I, alkyne, and alkene. For the unsubstituted precursors (27–30), only the benzoselnazole (27) was synthesized while the rest (28–30) were commercially available. They were then converted to the corresponding Br or I analogs (31–38), which were then converted to the alkyne or alkene analogs (39–47) by appropriate cross-coupling reaction. The corresponding alkene and alkyne selenium analogs (42) and (47), respectively) were synthesized as shown in scheme 3 from intermediate (26) through peptide coupling with the corresponding carboxylate, followed by deoxygenative cyclization with POCl3. In addition, a benzothiazole analog with an OTf group at the 2 position was synthesized and tested (figure S7A and scheme S7).
