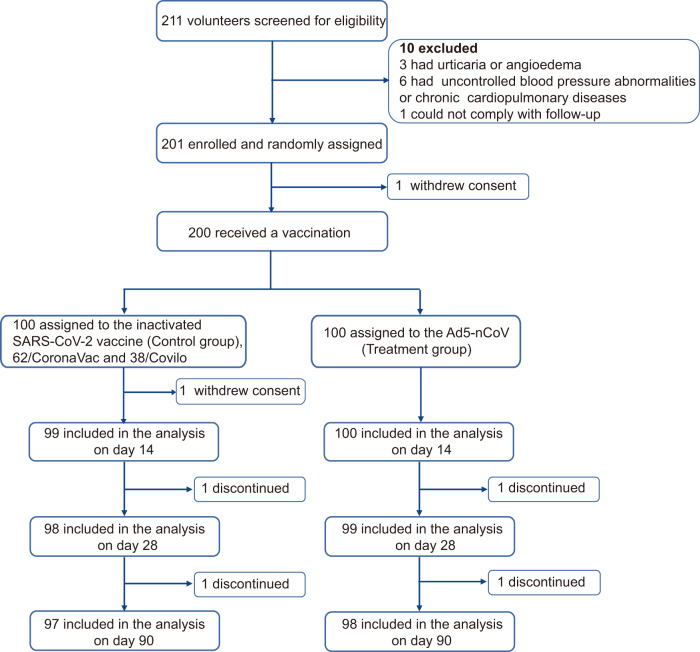
Fig. 1. Trial profile.
211 volunteers were recruited and screened for eligibility, among which 201 participants were enrolled and randomly assigned; 200 participants received the booster dose vaccination, and 1 participant refused to receive a vaccination after randomization. Participants (98 and 97) in the two groups completed the planned visits within 90 days after vaccination. One subject discontinued the trial on day 14 after vaccination (primary safety included up to that point). Four participants discontinued the follow-up visits but provided safety records up to day 28.