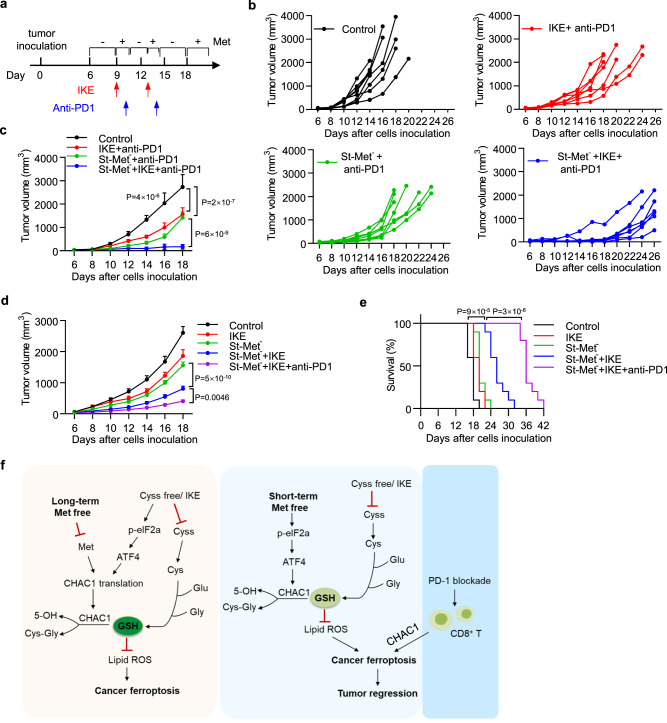
Fig. 8. Triple combination of dietary methionine intermittent starvation, IKE, and PD-1 blockade shows superior antitumor activity.
a–c Experimental design of B16F10 tumor in C57BL/6 mice (a). Tumor-bearing mice were fed with Met− diet intermittently (St-Met−) and treated with anti-PD-1 antibody (100 µg/mouse) alone or anti-PD-1 antibody plus IKE (20 mg/kg) at indicated days (a). Individual tumor growth was monitored over time (b). Tumor volumes of different treatment groups were plotted as mean ± s.e.m., n = 7 (IKE + anti-PD1, St-Met− + anti-PD1, and St-Met− + IKE + anti-PD1) or 8 (Control) mice per group and P values are determined by two-way ANOVA (c). d, e B16F10 tumor-bearing mice were fed with Met+ diet and treated with IKE (20 mg/kg), or fed with Met- diet intermittently (St-Met−) and treated with IKE (20 mg/kg) or IKE plus anti-PD-1 antibody (100 µg/mouse), n = 10 mice per group. Tumor volumes of different treatment groups were plotted as mean ± s.e.m., and P values were determined by two-way ANOVA (d). Kaplan–Meier survival curves of these animals (e) and P values are determined by log-rank test (e). f Schematic representation of the proposed model. Under long-term methionine deprivation, CHAC1 translation induced by cystine deprivation is blocked, resulting in reduced GSH degradation and impaired ferroptosis onset (left). Upon short-term methionine pre-starvation, CHAC1 transcription is stimulated, but translation is not inhibited, resulting in enhanced GSH degradation and ferroptosis sensitization. PD-1 blockade-activated CD8+ T cells synergize with dietary methionine intermittent deprivation to induce potent cancer cell ferroptosis and improved antitumor immunity (right). Source data are provided as a Source Data file.