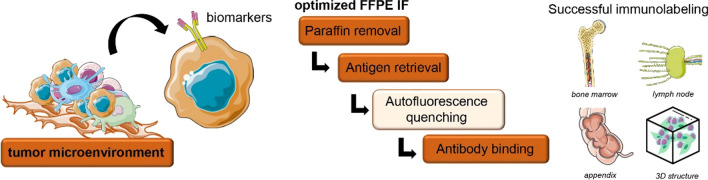
Abstract
The study of neoplastic cells enabled the discovery of important tumor-related biomarkers which resulted in new forms of early diagnosis, therapeutic options, and prognostic markers. Thus, immunofluorescence (IF), a high throughput imaging technology, represents a valuable method that enables the virtual characterization and localization of diverse cell types and targets, preserving tissue architecture and spatial surroundings. IF staining and analysis of formalin-fixed paraffin-embedded (FFPE) tissues are considered a challenge due to several difficulties, such as tissue autofluorescence, non-specific antibody binding, and image acquisition and quality. This study aimed to develop a multiplex-fluorescence staining technique with high-contrast and high-quality multiple-color images to enrich the investigation of important biomarkers. We present a robust optimized multiple-immunofluorescence procedure that reduced sample autofluorescence, enabled the use of simultaneous antibodies on the same sample, and showed super-resolution imaging through precise antigen localization. We illustrated the utility of this powerful method in FFPE neoplastic appendix, lymph node and bone marrow biopsies, and a 3D-coculture system, in which cells are enabled to grow and interact with their surroundings in all 3D dimensions. Our optimized multiple-immunofluorescence method represents a powerful tool for better understanding the complexity of tumor cells, characterizing cell populations and spatial localization, revealing predictive and prognostic biomarkers, and identifying immunologic phenotypes in a single and limited sample. This valuable IF protocol successfully enables tumor microenvironment profiling that could contribute to the study of cellular crosstalk and the niche, and to the identification of predictive biomarkers for neoplasms.
Graphical abstract
Keywords: Immunofluorescence, Phenotype, Immune cells, Cell-to-cell, Biomarkers
Introduction
The increase in life expectancy has resulted in higher exposure of individuals to various environmental risk factors. This increased longevity has also resulted in the emergence of malignant disorders, which generally have a slow, progressive and irreversible evolution, in addition to possible latency periods (Atella et al. 2019). The study of the neoplastic cells is crucial for the discovery of important tumor-related biomarkers that benefit in new forms of early diagnosis, therapeutic options, and prognostic markers (Nimse et al. 2016). Moreover, the tumor microenvironment exploration also proved useful in cancer biology, enabling the understanding of immune-escape mechanisms and could be exploited as new treatment targets (Behrmann et al. 2018). Ideally, biomarkers should be specific to a particular cancer type or cell population and can include genetic markers, proteins or cellular metabolic products. Of note, proteins are among the most important and powerful molecules, since they frequently act as disease effectors, prognostic indicators, and potential therapeutic targets. The crosstalk between malignant cells and the bone marrow (BM) microenvironment is a pivotal contributor to disease initiation, maintenance, progression, and recurrence, mainly in hematological neoplasms (Forte et al. 2019). It is conceivable that tumor-associated immune cells modulate the tumor-host interaction, which could lead to advances in the development of cancer immunotherapies and avoidance of graft versus host disease (Parra et al. 2017). To that end, a deeper characterization of the tumor microenvironment composition, cell-cell, cell-matrix, and co-localization interactions, are fundamental keys to understanding neoplasm complexities and to possibly improving cancer diagnosis, prognosis, and assertive treatment (Poltavets et al. 2018).
Immunohistochemistry (IHC), a method suitable for formalin-fixed paraffin-embedded (FFPE) samples has been routinely employed in surgical pathology laboratories. The standard IHC technique enables the characterization of a large variety of antigens, which still aids in the diagnosis of several types of malignant neoplasms (Magaki et al. 2019). However, significant drawbacks and pitfalls may arise during the steps of the IHC reaction, such as bad antigen recovery, heterogeneity of antibody staining between reactions, and non-specific background. The interpretation of the slides may be problematic as well due the subjective estimations that arise from visual inspection (Dubois et al. 2013). Also, the traditional IHC method considers one antigen at a time and does not allow the study of two or more antigens in the same reaction which could be especially useful to assess relationships between subtypes of immune and tumor cells. In this setting, immunofluorescence (IF) emerges as an additional method to explore the properties of biological samples (Ansari and Haqqi 2021), enabling the simultaneous identification of different cell types and permitting co-localization of antigens within cellular compartments. Hence, IF is a powerful method to better characterize cellular crosstalk in the tumor microenvironment of malignant neoplasms (Bhakdi and Thaicharoen 2018). Although IF is a good choice for tissues that have not been exposed to fixatives, such as frozen samples, the application of this method in routine FFPE samples remains to be optimized to address particular limitations, such as high background autofluorescence, non-specific antibody binding, and cross-species reactivity. There are unmet needs for better antigen retrieval, adequate fluorescence intensity, accurate imaging visualization, and reliable reproducibility (Tóth and Mezey 2007).
The goal of the present study was to optimize a multiple-staining immunofluorescence protocol and to verify the applicability of this method for studying the tumor microenvironment and understanding the complexity of tumor cells and their neighboring microenvironment. FFPE sections of biopsies and bone marrow-mimicking 3D-coculture system were stained with, at least, 2 different antibodies, and analyzed with high-contrast, high-quality, multiple-color, multispectral confocal image microscopy.
Methods
Samples processing
Tridimensional (3D)-coculture system
Using a scalpel, 3D-structure materials were cut into consistent size pieces (5 mm × 5 mm × 5 mm), hydrated with Phosphate Buffered Saline (PBS) buffer and transferred to one well of a 96-well plate. Next, HS-5/mesenchymal stromal cells (MSC) lineage (RRID:CVCL_3720) suspended in RPMI-1640 (Sigma) culture media containing 10% Fetal Bovine Serum (FBS; Gibco), glutamine (2mM; Sigma), penicillin (100 µg/mL; Sigma), streptomycin (100 µg/mL; Sigma), and amphotericin B (0.25 µg/mL; Gibco) were infused into the 3D-structure, which was then incubated overnight. Hematopoietic cells isolated from human bone marrow were infused into the 3D-structure containing HS-5/MSC and the structure was placed in a cell culture incubator for 7 days. All incubation steps occurred under the same conditions, at 37 °C and 5% CO2. Finally, each cell-infused 3D-structure was collected and processed for formalin fixation/paraffin embedding.
Samples
Bone marrow hematopoietic stem cells were obtained from one healthy donor (HD), one patient with myelodysplastic syndromes (MDS), and one patient with de novo acute myeloid leukemia (AML).
Biopsy specimens were collected from patients with the following diagnosis [tissue]: lymphoid hyperplasia (cecal appendix), classic Hodgkin’s lymphoma (lymph node), and AML (bone marrow).
Ethical consideration
This study was approved by the Human Ethics Committee of the State University of Campinas (CAAE 45254321.7.0000.5404 and 1110.0.146.000-11), in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). All patients signed informed consent forms. All samples from patients included in this study were collected at diagnosis.
Slide preparation for paraffin-embedded samples
Samples were fixed with 10% neutral-buffered formalin (Sigma-Aldrich/Merck) and stored under climate-controlled conditions for 24 h. The structures were then rinsed with distilled water for 1 h followed by dehydration with 70%, 80%, and 95% alcohol for 45 min each, plus three changes of 100% alcohol (Merck) for 1 h each. Next, samples were cleared by 2 changes of xylene (Merck) for 1 h each, immersed in three changes of paraffin for 1 h each, and embedded in a paraffin block.
Sequential 4 μm-thick sections from the FFPE blocks were cut, floated in a 60 °C water bath, and affixed in Platinum glass slides. For paraffin removal, the slides were heated at 110 °C for 40 min in a dry incubator, and were deparaffinized by three changes of xylene (Merck), for 10 min each. The slides were then dehydrated in 100%, 85%, 70%, and 50% ethanol, for 5 min each (Merck).
Immunofluorescence
Antigen Retrieval for IF analysis
A heat-induced epitope retrieval procedure was performed by incubating slides in Tris Base / EDTA buffer, pH 9.0, at 95 °C for 40 min. After reaching room temperature, slides were washed three times with PBS buffer.
Treatment to reduce autofluorescence for IF analysis
Autofluorescence was blocked by incubating slides in a buffer containing hydrogen peroxide 30% (Merck) at a final concentration of 4.4 M in methanol (Merck), for 10 min, followed by one PBS buffer wash for 10 min at − 20 °C.
Antibody staining protocols for IF analysis
Non-specific binding sites were blocked with 5% Bovine Serum Albumin (BSA) in PBS buffer for 1 h, followed by incubation with primary antibodies (Table 1) diluted in 3% BSA, overnight (16 h) at 4 °C. For negative controls, samples were incubated or not with an IgG isotype control (Mouse IgG isotype control (Invitrogen, #02-6502), a Rabbit IgG isotype control (Invitrogen, #02-6502) and a Goat IgG isotype control (Invitrogen, #02-6502) or 3% BSA. Primary antibodies were removed, and the slides were washed three times with 0.1% PBS-Tween buffer for 5 min each at room temperature. Slides were then incubated with fluorescent secondary antibodies, diluted in a fluorescent DNA intercalating dye 4′,6′-diamino-2-phenyl-indole (DAPI) (10,236,276,001, Sigma) for 1 h at room temperature. After incubation, slides were washed three times with 0.1% PBS-T buffer for 5 min and mounted using ProLong Gold antifade reagent with DAPI (P36930, ThemoFisher / Invitrogen).
Table 1.
Antibody specificity
| Antibody | Clonality | Reactivity | Host | Catalog | Company | Dilution | RRID |
|---|---|---|---|---|---|---|---|
| Anti-CD3 | Monoclonal | Human | Rabbit | BSB5146 | BioSB | 1:100 | – |
| Anti-CD4 | Monoclonal | Human | Rabbit | BSB5153 | BioSB | 1:100 | – |
| Anti-CD4-PE | Monoclonal | Human | Mouse | 555,348 | BD | 1:100 | AB_395753 |
| Anti-CD8 | Monoclonal | Human | Mouse | BSB5174 | BioSB | 1:100 | – |
| Anti-CD8-FITC | Monoclonal | Human | Mouse | 11-0087-42 | Invitrogen | 1:100 | AB_10557240 |
| Anti-CD30 | Monoclonal | Human | Mouse | ab23766 | Abcam | 1:100 | AB_447667 |
| Anti-CD45 | Polyclonal | Human | Rabbit | ab10559 | Abcam | 1:100 | AB_442811 |
| Anti-CD34 | Monoclonal | Human | Mouse | ab198395 | Abcam | 1:250 | AB_2889381 |
| Anti-CD90 / Thy1 | Monoclonal | Human | Rabbit | ab133350 | Abcam | 1:100 | AB_11155503 |
| Anti-Stat3 | Polyclonal | Human | Goat | ab5073 | Abcam | 1:100 | AB_304731 |
| Alexa Fluor 488 | Polyclonal | Mouse | Chicken | #A-21,200 | Invitrogen | 1:500 | AB_2535786 |
| Alexa Fluor 488 | Polyclonal | Rabbit | Chicken | #A-21,441 | Invitrogen | 1:500 | AB_2535859 |
| Alexa Fluor 555 | Polyclonal | Mouse | Donkey | #A-31,570 | Invitrogen | 1:1000 | AB_2536180 |
| Alexa Fluor 555 | Polyclonal | Rabbit | Donkey | #A-31,572 | Invitrogen | 1:1000 | AB_162543 |
| Alexa Fluor 633 | Polyclonal | Goat | Donkey | #A-21,082 | Invitrogen | 1:250 | AB_141493 |
Imaging and confocal microscopy
Samples were examined in the National Institute of Science and Technology on Photonics Applied to Cell Biology (INFABIC) at UNICAMP, University of Campinas, using a Zeiss LSM 780-NLO confocal on an Axio Observer Z.1 microscope (Carl Zeiss AG, Germany) using a 63×optical objective. Images were collected using 488/555nm laser lines for excitation and 520/570 emission filters for AlexaFluor 488 and AlexaFluor 520 fluorophores, respectively, with pinholes set to 1 airy unit for each channel, 1024 × 1024 image format, and 63×optical zoom. Z-stacks were acquired and 3D-rendered at maximum intensity projection. In the absence of primary antibodies, staining of secondary antibodies (negative controls) failed to produce any significant staining. Images acquired from the Zeiss LSM780-NLO were processed using Fiji Is Just ImageJ (Fiji). Images were automatically adjusted for high resolution. In order to analyze the expression of the markers, micrographs were selected randomly and cells from each sample were manually counted to quantify the percentage of labeled cells.
Results
Immunofluorescence method optimization
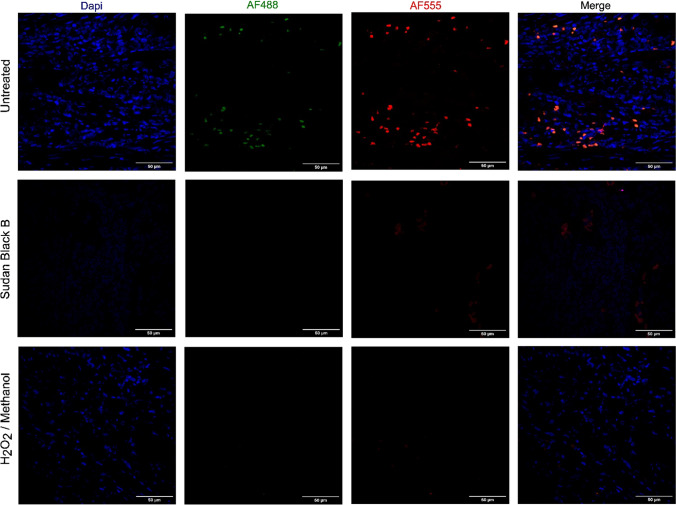
In order to analyze specific cell type identification, characterize cell-cell interaction, and use of two or more antigens, we developed and optimized a multiple IF in paraffin-embedded (FFPE) biopsy and 3D-coculture samples. As all samples were fixed using neutral-buffered formalin, in which protein crosslink is very common, we attempted to reduce the autofluorescence to avoid the mask of fluorophores specific label, impairing the quality and leading to false signals. After a heat-induced epitope retrieval process using a Tris/EDTA buffer with high pH levels (9.0), we verified that Sudan Black B dye yielded poor images in some samples while hydrogen peroxide plus methanol solution showed an important autofluorescence quenching in our FFPE samples (Fig. 1).
Fig. 1.
IF autofluorescence optimization. FFPE sections of adult human appendix were deparaffinized, rehydrated, subjected to antigen retrieval and treated with different reagents to reduce autofluorescence. Comparison of Untreated, Sudan Black B dye, and Peroxide Oxygen (H2O2) plus Methanol solution. Images were 3D-rendered at maximum intensity projection. Objective lens, 40×; N.A., 0.75; work distance, 1 mm. Scale bar: 50 μm
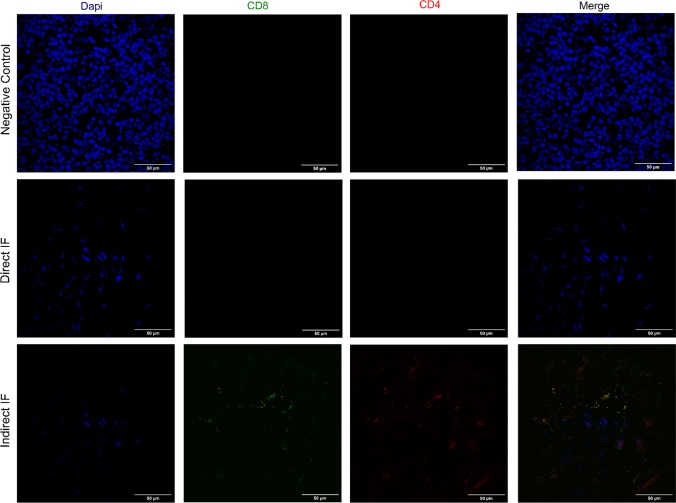
Following a non-target epitope blockage by BSA to prevent nonspecific antibody binding, antigen staining, direct or indirect, was carried out. We verified that high-quality 2-color fluorescence images were obtained from archival FFPE samples after indirect IF, using anti-CD8 and anti-CD4 as primary antibodies and 488 and 555-AlexaFluor dyes as secondary antibodies, respectively (Fig. 2).
Fig. 2.
Antigen Staining optimization of anti-CD8 and anti-CD4 primary antibodies. FFPE sections of adult human cecal appendix were deparaffinized, rehydrated and subjected to antigen retrieval. Sections were then stained with anti-CD8-FITC and CD4-PE or anti-CD8/Alexa Fluor 488-conjugated or anti-CD4 / Alexa Fluor 555-conjugated. Nuclei were counterstained with DAPI. Images were 3D-rendered at maximum intensity projection. Objective lens, 40×; N.A., 0.75; work distance, 1 mm. Scale bar: 50 μm
Immunofluorescence application
IF analysis of FFPE biopsies
To demonstrate the utility of our multiplex IF optimized method, we analyzed a 3D-coculture system and FFPE biopsies.
IF analysis of an FFPE 3D-coculture sample
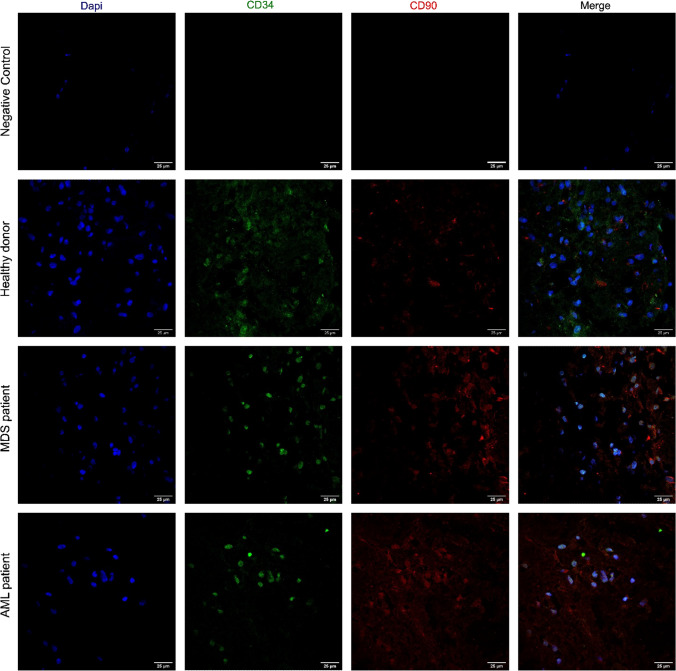
To test our optimized IF method, a 3D-co-culture system containing HS-5/MSC and primary hematopoietic cells isolated from bone marrow was used. After 7 days of coculture, a 3D-structure was collected, neutral-buffered formalin-fixed, paraffin-embedded, and IF was performed. Microscopy analysis enabled the observation of colocalization of hematopoietic cells (CD34-positive antigen) and HS-5/MSC (CD90-positive antigen), with a preserved cell morphology in these 3D-coculture systems (Fig. 3). Interestingly, both normal and malignant primary hematopoietic cells (CD34) were preferentially located in areas containing HS-5/MSC (Fig. 3), confirming the colocalization between these two cell types.
Fig. 3.
Multiple IF analysis of CD34 and CD90 antigens in a 3D-coculture system. FFPE sections of a 3D-coculture containing HS-5/MSC cocultured with CD34-positive cells isolated from the bone marrow of healthy donor, MDS and de novo AML patients were deparaffinized, rehydrated and subjected to antigen retrieval. Sections were stained with anti-CD34 (AlexaFluor 488-conjugated) or anti-CD90 (AlexaFluor 555-conjugated). Nuclei were counterstained with DAPI. Images were 3D-rendered at maximum intensity projection. Objective lens, 40×; N.A., 0.75; work distance, 1 mm. Scale bar: 25 μm
IF analysis of FFPE biopsies
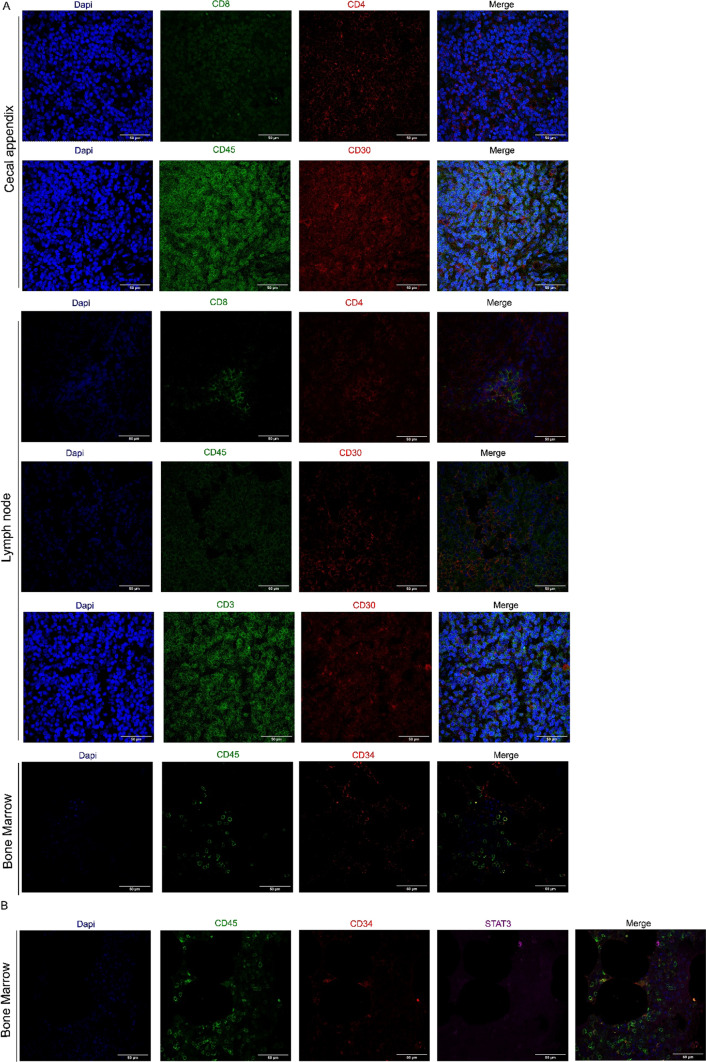
Next, we explored the differential cell antigen expression in FFPE biopsies of cecal appendix, lymph nodes, and bone marrow. In the cecal appendix, among the T-cell population, CD4 + T cells outweighed CD8 + T cells. We further observed a great frequency of CD45 + cells and a lower expression of CD30 + cells, a member of the tumor necrosis factor receptor superfamily. In lymph nodes, we observed a higher ratio of CD4 + T cells compared with CD8 + T cells. Additionally, we observed a higher ratio of CD45 + CD30 + cells compared with CD3 + CD30+. In the bone marrow, we observed a low frequency of CD34 + cells (Fig. 4A).
Fig. 4.
Multiple IF analysis of phenotype in cecal appendix, lymph nodes and bone marrow biopsies. FFPE sections were deparaffinized, rehydrated and subjected to antigen retrieval. A Cecal appendix, lymph nodes and bone marrow sections were stained with anti-CD4, CD30, CD34 (Alexa Fluor 555-conjugated) or anti-CD8, CD3, CD45 (Alexa Fluor 488-conjugated). B Bone marrow sections were stained with anti-CD45 (Alexa Fluor 488-conjugated), anti-CD34 (Alexa Fluor 555-conjugated) and anti-STAT3 (Alexa Fluor 633-conjugated). Nuclei were counterstained with DAPI. Images were 3D-rendered at maximum intensity projection. Objective lens, 40×; N.A., 0.75; work distance, 1 mm. Scale bar: 50 μm
After evaluation of the CD34 + population in the bone marrow, we verified that the immunofluorescence method could be helpful for the identification of prognostic and therapeutic markers for hematological malignancies, such as STAT3 (Fig. 4B).
Discussion
Immunofluorescence is a helpful method to virtually visualize cellular spatial localization, immunologic phenotypes, and several components, due to the combination of specific antibodies and fluorophores (Im et al. 2019). In comparison with IHC, multiple IF allows the analysis of more than two antigens, protein, and cell types in the same sample while preserving tissue architecture and spatial surroundings. Therefore, broad applications in the clinical and research fields since cells, tissues or entire organisms could be evaluated through IF. Nonetheless, IF protocols should be optimized in order to avoid autofluorescence or non-specific antibody binding, as well as to enhance antibody signal contrast since these processes can generate false positive results that, if not taken into account, may lead to wrong conclusions (Axelrod et al. 2018). In this setting, we optimized, validated, and described a protocol for generating high-contrast, high-quality, multi-color IF images suitable for image analysis from paraffin-embedded samples to better characterize antigen expression, cell type, and cellular localization.
Tissue fixation, the first step in an IF protocol, is an important process for preserving morphology and immunoreactivity, maintaining antigenicity, and preventing autolysis, with maximum access of antibodies to their targets. However, protein cross-linking with the formation of methylene bridges that mask the antigenic epitope target could be formed requiring an antigen retrieval processing (Bogen et al. 2009). For our FFPE samples, we verified that the heat-induced epitope retrieval preserved the structure and allowed the antigen exposure. We next attempted to reduce the tissue autofluorescence, since high levels in samples could entirely mask specific labeling with fluorophores, impair the quality of images and lead to false signals. Interestingly, the autofluorescence can be due to a range of factors, including intrinsic fluorescence of natural cell or tissue components (fibers, enzymes, cellular) (Croce and Bottiroli 2014) or fixative solutions (reaction between formaldehyde and amine groups) (Kajimura et al. 2016), which produce excessively fluorescent emission wavelengths between 420 and 650 nm (Viegas et al. 2007). We tested a light-absorbing dye, Sudan Black B reagent, which quenches autofluorescence from the natural tissue component, and a hydrogen peroxide/methanol solution, which oxidizes the molecules and complexes responsible for emitting fluorescence in the tissue. In our analysis, Sudan Black B dye yielded poor images while hydrogen peroxide/methanol solution showed better autofluorescence quenching.
After prevention of nonspecific antibody binding using BSA (Daneshtalab et al. 2010; Buchwalow et al. 2011), we optimized the antibody staining using the direct (with a labeled primary antibody) or indirect (with a primary unconjugated antibody, and a secondary fluorophore) IF methods. We verified that the use of a secondary antibody showed a higher sensitivity and signal amplification when compared to a primary-conjugated antibody. The secondary antibodies provided stable signal generation in imaging and showed sensitive detection with no significant self-quenching.
Studies have been performed for demonstrating the usage of immunofluorescence in several tissues. Sorrele and colleagues validated antibodies specific for mouse immune cell markers to distinguish B cells, T cells, NK cells, macrophages, dendritic cells, and neutrophils, and evaluated the immune microenvironment in a preclinical model of breast cancer (Sorrelle et al. 2019). Jia and colleagues described the tumor immune microenvironment features in gastric cancer, using patient samples (Jia et al. 2022). Thus, to demonstrate the applicability and utility of this immunofluorescence method, we analyzed FFPE biopsy samples of the neoplastic appendix, lymph node, and bone marrow, focusing on a better characterization of cell types and their contribution to diagnosis, immune response, and prognosis. In this setting, we choose to analyze antigen markers for hematopoietic cells (CD34), and immune cells, including major T-cells (CD3), T-helper cell subset (CD4), T-cytotoxic cell subset (CD8), activated T and B cells (CD30), and leukocyte common antigen marker (CD45). In the FFPE neoplastic appendix and lymph node biopsies, we verified a higher CD30 and CD45 antigen expression and a short cell-to-cell distance. Additionally, our IF analysis allowed the characterization of the most common immunophenotype in classical Hodgkin lymphoma, the expression of CD30 and CD45 (Gualco et al. 2012). These results demonstrate that the optimized IF method could help in this neoplasm diagnosis. Furthermore, in these same samples, CD4 and CD8 positive cells were located in the same spatial area, indicating a possible initiation of malignant cell elimination due to the fact that these antigens are expressed during the adaptative immune response. We further studied the CD3 expression, present in all Hodgkin lymphoma, which activates the CD30 antigen and consequently T-cell response. A short cell-to-cell distance between CD3 and CD30 positive cells was found, indicating that the IF method could be beneficial to study protein interactions, crosstalk, and immune response.
In FFPE bone marrow biopsies, we analyzed two important markers, CD34, a hematopoietic cell marker, and CD45, a leukocyte common antigen marker due to the fact that these markers are associated with poor prognosis in patients with hematological neoplasms (Sawadogo et al. 2013). We observed close spatial distribution between CD45 and CD34 positive cells, indicating that immature and mature hematopoietic cells might occupy the same niche space. To verify the cell interdependence, we further used the novel optimized IF method in a 3D-coculture system, containing pre-leukemic and leukemic stem cells (CD34 positive) and mesenchymal stromal cells (CD90 positive). We demonstrated that primary malignant hematopoietic stem cells were found nearby the mesenchymal stromal cells, indicating the contact dependence of hematopoietic stem cells to their surroundings, mainly with stromal cells. These results showed that the IF protocol could be valuable to analyze the microenvironment.
Moreover, as the search for therapeutic options against hematological neoplasms represents a challenge, the investigation of molecular targets becomes crucial for the development and improvement of novel pharmacological agents that exhibit specific effects against neoplastic cells (Sami et al. 2020). Hence, we additionally explored the expression of an important biological marker, STAT3, a regulatory factor of signal transduction and transcriptional activation that is associated with carcinogenesis and has been reported to inhibit apoptosis of neoplastic cells (Shi et al. 2018).
Conclusion
Our optimized and validated multiple immunofluorescence assay showed lower levels of autofluorescence and increased antigen exposition, enabling the analysis of the same FFPE section using, at least, two antibodies. This multiple IF optimization not only enables the identification of different targets/antigens in the same simple section but also represents a valuable method for tumor microenvironment profiling that could contribute to the study of cellular crosstalk and the niche, and to the identification of predictive and prognostic biomarkers for neoplasms. The application of multiple staining in the same section and the opportunity to generate a panel with several markers could be beneficial to scientific research as well as clinical analysis. Furthermore, the application of this method is valuable to better understand the complexity of tumor cells, characterize cell populations and spatial localization, discover predictive and prognostic biomarkers, identify immunologic phenotypes and determine localization in a unique spatial system. Therefore, this multiple IF method could contribute to deeper investigation and elucidation of the tumor surroundings, contributing to the improvement of neoplasm diagnosis, and prognosis, and guide future treatments.
Despite the fact that this novel method proved to be efficient and powerful in providing reproducible, and high-quality staining, it should be underlined that for accurate immunofluorescence results rigorous steps must be followed. The key steps are (1) careful IF assay design, choosing the best combination of antibodies and generating a panel of markers to use in the multiple IF; (2) the use of fresh or recently cut tissue sections to prevent poor tissue morphology and staining; (3) select appropriate negative and secondary control samples; (4) select an antigen retrieval process that allows the binding of the antibodies to the epitope; (5) optimization of the autofluorescence conditions due to the fact that this process could mask specific labeling with fluorophores; (6) optimization and validation of antibodies by IHC or IF; and (7) use of confocal microscopy image analysis to detect a minimum of staining.
Acknowledgements
The authors would like to thank Raquel S Foglio (Hematology and Hemotherapy Center, University of Campinas, São Paulo, Brazil) for the English revision; Pedro Augusto Silva Nogueira and Licio Augusto Velloso (Obesity and Comorbidities Research Center (OCRC) - University of Campinas-Unicamp, Campinas, São Paulo, Brazil) for immunofluorescence assistance. We also thank the National Institute of Science and Technology on Photonics Applied to Cell Biology (INFABIC) at UNICAMP for the access to equipment and confocal assistance and analysis (FAPESP grants 2014/50938-8 and CNPq grants 465699/2014-6). The authors also thank CNPq and FAPESP for the financial support.
Author contributions
1 and 2 designed and performed the experiments, collected, analyzed and interpreted data, and wrote the manuscript; 3 designed the experiments, analyzed and interpreted immunofluorescence data; 4 analyzed immunofluorescence data; 1 and 5 directed the research; 5 provided financial support, revised and gave final approval of the manuscript. All authors have read, commented and approved the final version of the manuscript.
Funding
This study was funded by Fundação de Amparo a Pesquisa do Estado de São Paulo (Grants #2017/21801-2, #2019/25247-5 and #2021/05320-0, São Paulo Research Foundation (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ansari MY, Haqqi TM. Assessing chondrocyte status by immunofluorescence-mediated localization of parkin relative to Mitochondria. Methods Mol Biol. 2021;2245:215–224. doi: 10.1007/978-1-0716-1119-7_15. [DOI] [PubMed] [Google Scholar]
- Atella V, Piano Mortari A, Kopinska J, et al. Trends in age-related disease burden and healthcare utilization. Aging Cell. 2019;18:e12861. doi: 10.1111/acel.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod HD, Pienta KJ, Valkenburg KC. Optimization of immunofluorescent detection of bone marrow disseminated Tumor cells. Biol Proced Online. 2018;20:13. doi: 10.1186/s12575-018-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann L, Wellbrock J, Fiedler W. Acute myeloid leukemia and the bone marrow niche-take a closer look. Front Oncol. 2018;8:444. doi: 10.3389/fonc.2018.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi SC, Thaicharoen P. Easy Employment and Crosstalk-Free detection of seven fluorophores in a Widefield fluorescence microscope. Methods Protoc. 2018 doi: 10.3390/mps1020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen SA, Vani K, Sompuram SR. Molecular mechanisms of antigen retrieval: antigen retrieval reverses steric interference caused by formalin-induced cross-links. Biotech Histochem. 2009;84:207–215. doi: 10.3109/10520290903039078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwalow I, Samoilova V, Boecker W, Tiemann M. Non-specific binding of antibodies in immunohistochemistry: fallacies and facts. Sci Rep. 2011;1:28. doi: 10.1038/srep00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce AC, Bottiroli G. Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis. Eur J Histochem. 2014;58:2461. doi: 10.4081/ejh.2014.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshtalab N, Doré JJ, Smeda JS. Troubleshooting tissue specificity and antibody selection: procedures in immunohistochemical studies. J Pharmacol Toxicol Methods. 2010;61:127–135. doi: 10.1016/j.vascn.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Dubois L, Andersson K, Asplund A, Björkelund H. Evaluating real-time immunohistochemistry on multiple tissue samples, multiple targets and multiple antibody labeling methods. BMC Res Notes. 2013;6:542. doi: 10.1186/1756-0500-6-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte D, Krause DS, Andreeff M, et al. Updates on the hematologic tumor microenvironment and its therapeutic targeting. Haematologica. 2019;104:1928–1934. doi: 10.3324/haematol.2018.195396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualco G, Natkunam Y, Bacchi CE. The spectrum of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma: a description of 10 cases. Mod Pathol. 2012;25:661–674. doi: 10.1038/modpathol.2011.200. [DOI] [PubMed] [Google Scholar]
- Im K, Mareninov S, Diaz MFP, Yong WH. An introduction to performing immunofluorescence staining. Methods Mol Biol. 2019;1897:299–311. doi: 10.1007/978-1-4939-8935-5_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K, Chen Y, Sun Y, et al. Multiplex immunohistochemistry defines the tumor immune microenvironment and immunotherapeutic outcome in CLDN18.2-positive gastric cancer. BMC Med. 2022;20:223. doi: 10.1186/s12916-022-02421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura J, Ito R, Manley NR, Hale LP. Optimization of single- and dual-color immunofluorescence protocols for Formalin-Fixed, paraffin-embedded archival tissues. J Histochem Cytochem. 2016;64:112–124. doi: 10.1369/0022155415610792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaki S, Hojat SA, Wei B, et al. An introduction to the performance of immunohistochemistry. Methods Mol Biol. 2019;1897:289–298. doi: 10.1007/978-1-4939-8935-5_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimse SB, Sonawane MD, Song KS, Kim T. Biomarker detection technologies and future directions. Analyst. 2016;141:740–755. doi: 10.1039/c5an01790d. [DOI] [PubMed] [Google Scholar]
- Parra ER, Uraoka N, Jiang M, et al. Validation of multiplex immunofluorescence panels using multispectral microscopy for immune-profiling of formalin-fixed and paraffin-embedded human tumor tissues. Sci Rep. 2017;7:13380. doi: 10.1038/s41598-017-13942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltavets V, Kochetkova M, Pitson SM, Samuel MS. The role of the Extracellular Matrix and its Molecular and Cellular regulators in Cancer Cell plasticity. Front Oncol. 2018;8:431. doi: 10.3389/fonc.2018.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami SA, Darwish NHE, Barile ANM, Mousa SA. Current and future molecular targets for Acute myeloid leukemia therapy. Curr Treat Options Oncol. 2020;21:3. doi: 10.1007/s11864-019-0694-6. [DOI] [PubMed] [Google Scholar]
- Sawadogo D, Tolo A, Kassi H, et al. Interest in determining the CD34 + CD38- phenotype in the diagnosis and prognosis of Acute Leukemia in Abidjan - Côte d’Ivoire. Mediterr J Hematol Infect Dis. 2013;5:e2013023. doi: 10.4084/MJHID.2013.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Zhang Z, Qu X, et al. Roles of STAT3 in leukemia (review) Int J Oncol. 2018;53:7–20. doi: 10.3892/ijo.2018.4386. [DOI] [PubMed] [Google Scholar]
- Sorrelle N, Ganguly D, Dominguez ATA, et al. Improved Multiplex immunohistochemistry for Immune Microenvironment evaluation of mouse Formalin-Fixed, paraffin-embedded tissues. J Immunol. 2019;202:292–299. doi: 10.4049/jimmunol.1800878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth ZE, Mezey E. Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J Histochem Cytochem. 2007;55:545–554. doi: 10.1369/jhc.6A7134.2007. [DOI] [PubMed] [Google Scholar]
- Viegas MS, Martins TC, Seco F, do Carmo A. An improved and cost-effective methodology for the reduction of autofluorescence in direct immunofluorescence studies on formalin-fixed paraffin-embedded tissues. Eur J Histochem. 2007;51:59–66. [PubMed] [Google Scholar]