Abstract
Abstract
Discoidin domain receptor 1 (DDR1) is one of the receptors that belong to a family of non-integrin collagen receptors. In common, DDR1 is predominantly found in epithelial and smooth muscle cells and its mainly involved in organogenesis during embryonic development. However, it’s also overexpressed in several pathological conditions, including cancer and inflammation. The DDR1 is reported in numerous cancers, including breast, prostate, pancreatic, bladder, lung, liver, pituitary, colorectal, skin, gastric, glioblastoma, and inflammation. DDR1 activates through the collagen I, IV, IGF-1/IGF1R, and IGF2/IR, regulating downstream signaling molecules such as MAPKs, PI3K/Akt, and NF-kB in diseases. Despite its biomedical importance, there is a lack of consolidated network map of the DDR1 signaling pathway, which prompted us for curation of literature data pertaining to the DDR1 system following the NetPath criteria. We present here the compiled pathway map comprises 39 activation/inhibition events, 17 catalysis events, 22 molecular associations, 65 gene regulation events, 35 types of protein expression, and two protein translocation events. The detailed DDR1 signaling pathway map is made freely accessible through the WikiPathways Database (https://www.wikipathways.org/index.php/ Pathway: https://www.wikipathways.org/index.php/Pathway:WP5288).
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12079-022-00714-x.
Keywords: Cancer, Inflammation, Post-translational modifications, Protein–protein interactions, Signaling pathways, WikiPathways
Introduction
Discoidin domain receptor 1 (DDR1) belongs to a family of two non-integrin collagen receptors and shows tyrosine kinase activity (Shrivastava et al. 1997; Alves et al. 1995). It was first discovered by Johnson et al. in 1993 (Johnson et al. 1993). Collagens were identified as their physiological ligands (Shrivastava et al. 1997; Vogel et al. 1997). DDR1 is a widely studied receptor tyrosine kinase, predominantly expressed in normal epithelial cells. The DDR1 gene encodes on chromosome 6 (6p21.3) in humans and is composed of five exons, which undergo alternative splicing to form five different isoforms, i.e., from DDR1a to DDR1e (Dorison et al. 2017). DDR1 has been found in human bronchial epithelial cells, keratinocytes, colon epithelial cells, liver, cornea, and vascular smooth muscle cells, as well as developing neuroectoderm, mammary glands, brain, distal tubule, podocytes, and smooth muscle cells in mice (Gross et al. 2004; Vogel et al. 2006). DDR1 is activated in a variety of diseases to contribute to proliferation, inflammation, and fibrosis (Dorison et al. 2017).
The aberrant expression of DDR1 is associated with tumor progression in a variety of human cancers, including breast, lung, ovary, liver, gastric, and glioma (Weiner et al. 2000; Heinzelmann-Schwarz et al. 2004; Yamanaka et al. 2006; Park et al. 2007; Shen et al. 2010; Ambrogio et al. 2016; Jin et al. 2018). Several studies have reported that the role of DDR1 depends on the type and stage of the cancer; its overexpression promotes tumorigenesis and is associated with a poor prognosis (Valiathan et al. 2012; Borza and Pozzi 2014). DDR1 promotes the epithelial-mesenchymal transition in cancer cells and it can be used as a biomarker in renal cancer (Song et al. 2016). In addition, DDR1 plays a key role in pro-inflammatory and profibrotic processes in atherosclerosis (Franco et al. 2009; Dorison et al. 2017). It also plays a key role in the development of permanent pulmonary epithelial lesions in idiopathic pulmonary fibrosis (Chen et al. 2016).
DDR1 expression is related to the onset and progression of chronic kidney disease (Flamant et al. 2006). Its expression and activation in macrophages are required for efficient migration, indicating a role for DDR1 in mediating interstitial lesions caused by macrophage infiltration in the kidney (Guerrot et al. 2011). The collective evidence of several reports suggests that DDR1 is a potential therapeutic target, as it is involved in organ fibrosis and several cancers. DDR1 activation or overexpression induces major downstream signaling pathways such as PI3K/AKT/mTOR, MAPK1/3, MAPK14, MAPK8/9, NF-KB (Matada et al. 2021).
The inhibition of DDR1 receptor tyrosine kinase by inhibitors may provide an appealing strategy to treat the diseases. For example, several multi-target kinase inhibitors, including imatinib, dasatinib, and nilotinib, are being used to inhibit downstream signalings mediated by DDR1 (Kothiwale et al. 2015). In recent years, a panel of selective DDR1 kinase inhibitors such as DDR1‐IN‐1 and 7rh benzamide, which inhibit the kinase activity of DDR1 has been developed for the treatment of cancer including colorectal cancer cells and lung adenocarcinoma (Tao et al. 2019). The importance of DDR1 signaling in a variety of pathological conditions especially in different cancers and inflammation led us to create a DDR1 signaling pathway map. We created a signaling pathway map by literature mining to compile the molecular interactions between DDR1 and its ligand collagen. These have been systematically brought together through manual annotation of research articles available in the literature, allowing them to be represented as a single pathway map. The DDR1 signaling pathway map is made available through the WikiPathways database.
Methodology
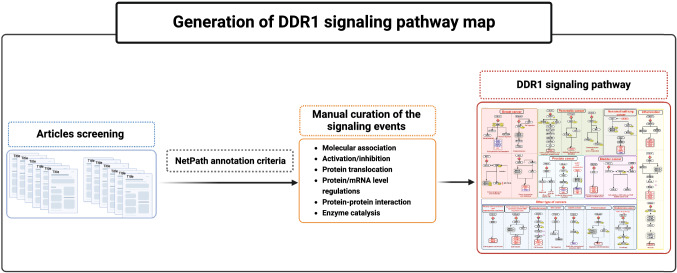
We performed a literature search in PubMed for research articles pertaining to DDR1-mediated signaling to develop a consolidated pathway map of DDR1. The scientific articles were obtained from PubMed using the following search terms (“DDR1” OR “CAK” OR “CD167” OR “DDR” OR “EHGK2” OR “MCK10” OR “NEP” OR “NTRK4” OR “PTK3” OR “PTK3A” OR “RTK6” OR “TRKE” OR “discoidin domain receptor tyrosine kinase 1”) AND ("pathway" OR "signaling" OR "signalling"). The abstract of each research article was manually screened to select only articles relevant to DDR1-mediated signaling events. Signaling reactions were manually curated according to the previously published NetPath annotation criteria (Kandasamy et al. 2010). The curated reactions mediated by DDR1 signaling were categorized into five groups (1) molecular associations, (2) catalysis/post-translational modifications (PTMs), (3) protein-activation/inhibition, (4) protein/gene regulations, and (5) protein translocation between cell organelles. Additional information, including cell lines used in the experiment, experiment type, and the information about sites and residues of PTMs were also curated. Each molecular event described in the DDR1 signaling pathway was linked to the PubMed entry of the corresponding articles. Manual curation of signaling events was enabled using a manual curation software called PathBuilder (Kandasamy et al. 2009). PathVisio was used to depict and visualize the signaling pathway map (Kutmon et al. 2015).
Results and discussion
The query terms searched in PubMed retrieved a total of 105 articles, which are pertinent to DDR1 mediated signaling. The manual screening of these articles provided 56 articles, which had specific information on DDR1 mediated signaling. The manual annotation of these selected articles provided 39 activation/inhibition events, 16 enzyme catalysis, 35 protein expression events, 65 gene regulation events, 22 molecular association and 2 translocation events (Supplementary Data S1). These events were compiled into a comprehensive pathway map of DDR1 signaling and made freely accessible through WikiPathways with the ID (Fig. 1).
Fig. 1.
Schematic representation of DDR1-mediated signaling pathway. Schematic representation of DDR1 induced signaling reactions. The signaling pathway map represents molecules involved in ligand-receptor interactions and DDR1 activated downstream molecular events including molecular association, enzyme catalysis, translocation, and gene regulation events. Information regarding the post-translational modification site and the residue is also shown in the pathway
A brief summary of DDR1 – mediated signaling map
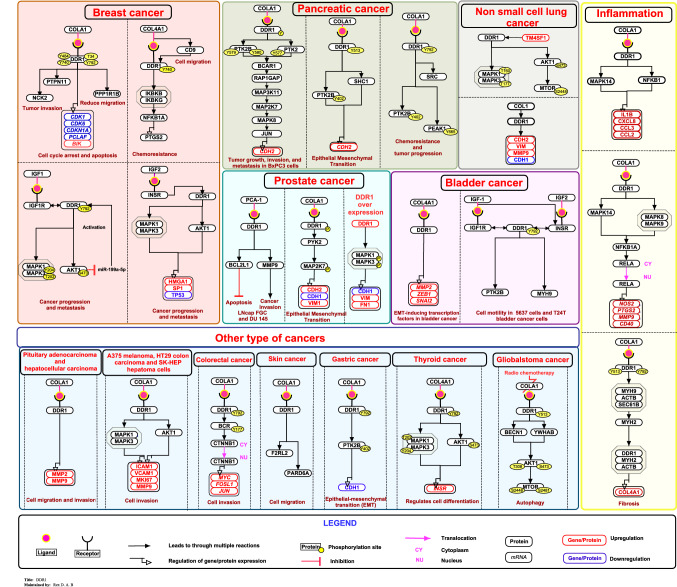
DDR1 is involved in many physiological and pathological conditions such as proliferation, inflammation and fibrosis (Dorison and Chantziantoniou 2018). The different expression levels of DDR1 and its complex effects in tumor progression has been widely reported in several types of human cancer, including breast cancer, renal clear cell carcinoma, non-small cell lung carcinoma, esophageal cancer, astrocytoma, prostate cancer, hepatocellular carcinoma and Hodgkin's lymphoma (Nemoto et al. 1997; Willenbrock et al. 2006; Shimada et al. 2008; Shen et al. 2010; Yang et al. 2010; Quan et al. 2011; Nakada et al. 2013; Toy et al. 2015; Song et al. 2016).
Breast cancer
In breast cancer cells, collagen I induce the phosphorylation of DDR1 at Tyr 484 and 740, interacting with NCK2 and PTPN11, respectively. This report demonstrated that NCK2 and PTPN11, as direct interaction partners of DDR1, play a physiological role in mammary gland development and during tumor invasion (Koo et al. 2006). Hansen et al. (2006) have reported that the phosphorylation of PPP1R1B (Dopamine and cAMP-regulated neuronal phosphoprotein, molecular mass 32 kDa, DARPP-32) at Thr 34 by the activation of DDR1 through collagen I reduces the migration of breast tumor cells (Hansen et al. 2006). The collagen IV-induced activation of DDR1 enhanced the PTGS2 expression, improving chemoresistance by activating the NF-kB pathway through IKBKB and IKBKG activation in breast cancer cells (Das et al. 2006). Collagen IV induces cell migration through the DDR1 and CD9-dependent pathway in MDA-MB-231 cancer cells (Castro-Sanchez et al. 2010). Collagen I induces DDR1 phosphorylation at Tyr 792 and attenuated mRNA expression of cell dependent kinases (CDK) CDK1, CDK6, CDKN1A, and KIAA0101, which enhanced BIK (BCL2 Interacting Killer) expression in cell cycle arrest and apoptosis in MCF-7 cells (Assent et al. 2015). The stimulation with IGF-1, induce the DDR1 overexpression and association with IGF-1R, which phosphorylates DDR1 at the Tyr 792 and activates AKT1 and MAPK1/3 pathways, which promotes the cancer progression in MCF-7 cells (Malaguarnera et al. 2015). The study in MCF-1 cells, IGF-1 stimulation induces DDR1 overexpression that activates IGF1R, PIK3CA/AKT1, and MAPK1/3 pathways that enhance cell growth and migration. AKT1 attenuated the expression of miR-199a-5p, which in turn leads to the upregulation of DDR1. This result demonstrated that the AKT / miR-199a-5p/DDR1 pathway plays a role in cell migration and proliferation in response to IGF-I (Mata et al. 2016). Vella et al (2017) have reported that insulin and IGF-2 activates insulin receptor (IR), which associate with DDR1 and induces the activation of the MAPK1/3 and AKT1 pathways, thereby regulating the upregulation of HMGA1, SP1, IR, and downregulation of TP53 which are involved in cancer progression and metastasis in breast cancer cells. Due to the overexpression of DDR1, which upregulates IR and downregulates p53 in breast cancer cells (Vella et al. 2017).
Prostate cancer
The overexpression of prostate cancer antigen (PCA)-1 contributes in the inhibition of apoptosis and invasiveness of human prostate cancer cells through the expression of BCL2L1 and MMP9 mediated by DDR1 (Shimada et al. 2008). Azizi et al (2019) have reported that collagen I induces autophosphorylation of DDR1 in prostate cancer cells LNcap‐FGC and DU145, which in turn induces the phosphorylation of the proteins such as PYK2 and MKK7 (MAP2K7) and promotes epithelial-to-mesenchymal transition (EMT) through enhanced expression of CDH2 and vimentin and induces migration by the attenuated expression of CDH1 (Azizi et al. 2019). In agreement with this, Zhao et al (2021) have reported that the overexpression of DDR1 promotes the EMT phenotype by increasing the expression of vimentin and fibronectin and down-regulation of CDH1 via the MAPK1/3 pathway in prostate cancer (Zhao et al. 2021).
Pancreatic cancer
In pancreatic cancer cells, phosphorylation of DDR1 at Tyr residue by collagen 1 induces the formation of a complex with PTK2B and BCAR1 and activation of RAP1GAP, MAP3K11, MAP2K7, MAPK8, JUN, and up-regulation of CDH2 protein, which are involved in tumor growth, invasion, and metastasis (Shintani et al. 2008). Huang et al (2016) reported that collagen I stimulates DDR1 phosphorylation at Tyr 513 and induces the up-regulation of CDH2 through the interaction between SHC1, phosphorylated PTK2B (Tyr 402), and promoted EMT in pancreatic cancer cells (Huang et al. 2016). The study by Aguilera et al (2017) reported that collagen I mediated activation of DDR1 induces the activation of SRC (Tyr 416), PEAK1 (Tyr 665), and PTK2B (Tyr 402), which are potentially responsible for collagen-induced chemoresistance and tumor progression in pancreatic cancer cells (Aguilera et al. 2017).
Bladder cancer
The overexpression of collagen IV and DDR1 in bladder cancer cells enhances cell motility and invasion and facilitates EMT-inducing transcription factors through enhanced expression of MMP2, ZEB1, and SLUG (Xie et al. 2020). DDR1 is phosphorylated by IGF1 and IGF2 at Tyr 792, forms a complex between PTK2B, MYH9, and IGFIR and induces cancer cell motility by regulating actin skeleton dynamics in bladder cancer cells (Buraschi et al. 2020).
Lung cancer
In Non-small-cell lung cancer cells (NSCLC), the overexpression of TM4SF1 interacts with DDR1 and promotes cancer proliferation, invasion and chemoresistance by activating AKT / MTOR pathway (Ye et al. 2019). Collagen I induces the upregulation of DDR1 and promotes cell invasion via EMT by enhancing the expression of CDH2, VIM, and MMP9 and attenuated CDH1 in A549 cells (Miao et al. 2013).
Other cancers
The overexpression of DDR1 by collagen I in pituitary adenocarcinoma and hepatocellular carcinoma cells mediates cell invasion by increasing the expression of MMP-2 and MMP-9 (Park et al. 2007; Yoshida and Teramoto 2007a, b). DDR1 phosphorylation by collagen I induces the activation of AKT1 and MAPK1/3 pathways and regulates the expression of ICAM1, VCAM1, MKI67, MMP9, and promotes invasion in human melanoma, colon carcinoma, and hepatoma cells (Romayor et al. 2020). In colorectal cancer cells, collagen I-mediated DDR1 phosphorylation at Tyr 792 induces the phosphorylation of BCR at Tyr 177, which disrupts the BCR / CTNNB1 interaction, resulting in an increased CTNNB1 nuclear activity that leads to the expression of target genes including MYC, FRA1, and JUN necessary for cell invasion (Jeitany et al. 2018). In skin cancer cells, DDR1 forms a complex with F2RL2 and PARD6A, which controls the localization of RhoE to cell–cell contacts, where it suppresses actinomyosin contractility, and promotes collective cell migration (Hidalgo-Carcedo et al. 2011). Hur et al (2017) have reported that collagen I/DDR1 signaling promotes tumor aggressiveness and EMT in gastric cancer by the phosphorylation of DDR1 at Tyr 792, PYK2 in Tyr 402 and reduced expression of CDH1(Hur et al. 2017). DDR1 overexpression increases IR protein and gene expression, which in turn increases the phosphorylation of AKT1 at Ser 473 and MAPK1/3 in Thr 202/Tyr 204, which regulates cell differentiation through the IGF-2 / IR-1 autocrine signaling loop in anaplastic thyroid cancer cells (Vella et al. 2019). In glioblastoma, collagen I stimulates DDR1 phosphorylation and forms a complex with YWHAB, BECN1, and AKT1 which mediates AKT1 and MTOR signaling to regulate the sensitivity of autophagy-associated therapy (Vehlow et al. 2019).
Inflammation and fibrosis
Collagen I phosphorylates DDR1 at Tyr 513 and Tyr 792 and forms a DDR1/MYH9/ACTB complex that interacts with SEC61B in the endoplasmic reticulum (ER). This interaction promotes nuclear translocation of DDR1 and its association with chromatin where they interacts with transcription factors MYH9 and ACTB and enhances the transcription of collagen IV and promotes fibrosis in the proximal tubules of the injured human and mouse kidneys (Chiusa et al. 2019). Collagen I/DDR1 interaction in human macrophages enhances the production of IL-1B, CXCL8, CCL3, and CCL2 via activation of MAPK14, and NFKB pathways, providing a novel mechanism by which macrophages produce and release large amounts of these chemokines in a tissue microenvironment in the course of inflammatory responses (Matsuyama et al. 2004). Collagen I stimulates DDR1 activation and induces NO production through NOS2 expression, and activation of RELA, MAPK14, and MAPK 8/9 pathways induces an increased inflammatory response in J774 murine macrophages (Kim et al. 2007). Seo et al (2008) reported that collagen promotes inflammation in the brain by stimulating microglial brain cells through the activation of DDR1, which induces upregulation of NOS2, PTGS2, CD40, and MMP9 via RELA, MAPK14, and MAPK 8/9 pathways perpetuating neuroinflammation (Seo et al. 2008). DDR1 stimulated by COL1A1 and COL8A1 phosphorylates DDR1 and stimulates the expression of MMP2 and MMP9 in rat injured artery smooth muscle cells, which induces proliferation, migration and invasion during arterial wound repair (Hou et al. 2001).
Conclusions
The DDR1-mediated signaling is involved in various cellular processes including cell proliferation, invasion, migration, differentiation, and cytokine expression. It is highly expressed in malignancies compared to normal cells. This suggested that DDR1 might be a potential target for cancer management. Recently, a panel of selective DDR1 kinase inhibitors has been developed, such as DDR1IN1 and 7rh benzamide, which inhibit DDR1 kinase activity for treating diseases including cancer. Therefore, the accessibility of DDR1-mediated signaling in the public resource will help biomedicine researchers to understand the role of DDR1 in the progression of diseases. We believe that this resource will provide a platform to the scientific community to identify novel therapeutic drug targets for cancer associated with DDR1 signaling.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Karnataka Biotechnology and Information Technology Services (KBITS), Government of Karnataka, for the support to the Center for Systems Biology and Molecular Medicine at Yenepoya (Deemed to be University) under the Biotechnology Skill Enhancement Programme in Multiomics Technology (BiSEP GO ITD 02 MDA 2017).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shobha Dagamajalu and D. A. B. Rex have Contributed equally to this work.
Contributor Information
Shobha Dagamajalu, Email: shobha_d@yenepoya.edu.in.
D. A. B. Rex, Email: rexprem@yenepoya.edu.in
G. P. Suchitha, Email: suchithajrf@yenepoya.edu.in
Akhila B. Rai, Email: akhilajrf@yenepoya.edu.in
Shreya Kumar, Email: shreyasunilkumar911@gmail.com.
Shreya Joshi, Email: shreyaj1400@gmail.com.
Rajesh Raju, Email: rajeshraju@yenepoya.edu.in.
T. S. Keshava Prasad, Email: keshav@yenepoya.edu.in.
References
- Aguilera KY, Huang H, Du W, Hagopian MM, Wang Z, Hinz S, Hwang TH, Wang H, Fleming JB, Castrillon DH, Ren X, Ding K, Brekken RA. Inhibition of discoidin domain receptor 1 reduces collagen-mediated tumorigenicity in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2017;16(11):2473–2485. doi: 10.1158/1535-7163.MCT-16-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves F, Vogel W, Mossie K, Millauer B, Ullrich A (1995) Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene 10(3): 609–618 [PubMed]
- Ambrogio C, Gomez-Lopez G, Falcone M, Vidal A, Nadal E, Crosetto N, Blasco RB, Fernandez-Marcos PJ, Sanchez-Cespedes M, Ren X, Wang Z, Ding K, Hidalgo M, Serrano M, Villanueva A, Santamaria D, Barbacid M. Combined inhibition of DDR1 and Notch signaling is a therapeutic strategy for KRAS-driven lung adenocarcinoma. Nat Med. 2016;22(3):270–277. doi: 10.1038/nm.4041. [DOI] [PubMed] [Google Scholar]
- Assent D, Bourgot I, Hennuy B, Geurts P, Noel A, Foidart JM, Maquoi E. A membrane-type-1 matrix metalloproteinase (MT1-MMP)-discoidin domain receptor 1 axis regulates collagen-induced apoptosis in breast cancer cells. PLoS ONE. 2015;10(3):e0116006. doi: 10.1371/journal.pone.0116006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi R, Salemi Z, Fallahian F, Aghaei M. Inhibition of didscoidin domain receptor 1 reduces epithelial-mesenchymal transition and induce cell-cycle arrest and apoptosis in prostate cancer cell lines. J Cell Physiol. 2019;234(11):19539–19552. doi: 10.1002/jcp.28552. [DOI] [PubMed] [Google Scholar]
- Borza CM, Pozzi A. Discoidin domain receptors in disease. Matrix Biol. 2014;34:185–192. doi: 10.1016/j.matbio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buraschi S, Morcavallo A, Neill T, Stefanello M, Palladino C, Xu SQ, Belfiore A, Iozzo RV, Morrione A. Discoidin domain receptor 1 functionally interacts with the IGF-I system in bladder cancer. Matrix Biol plus. 2020;6–7:100022. doi: 10.1016/j.mbplus.2020.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Sanchez L, Soto-Guzman A, Navarro-Tito N, Martinez-Orozco R, Salazar EP. Native type IV collagen induces cell migration through a CD9 and DDR1-dependent pathway in MDA-MB-231 breast cancer cells. Eur J Cell Biol. 2010;89(11):843–852. doi: 10.1016/j.ejcb.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Chen C, Deng J, Yu X, Wu F, Men K, Yang Q, Zhu Y, Liu X, Jiang Q. Identification of novel inhibitors of DDR1 against idiopathic pulmonary fibrosis by integrative transcriptome meta-analysis, computational and experimental screening. Mol Biosyst. 2016;12(5):1540–1551. doi: 10.1039/C5MB00911A. [DOI] [PubMed] [Google Scholar]
- Chiusa M, Hu W, Liao HJ, Su Y, Borza CM, de Caestecker MP, Skrypnyk NI, Fogo AB, Pedchenko V, Li X, Zhang MZ, Hudson BG, Basak T, Vanacore RM, Zent R, Pozzi A. The extracellular matrix receptor discoidin domain receptor 1 regulates collagen transcription by translocating to the nucleus. J Am Soc Nephrol. 2019;30(9):1605–1624. doi: 10.1681/ASN.2018111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Ongusaha PP, Yang YS, Park JM, Aaronson SA, Lee SW. Discoidin domain receptor 1 receptor tyrosine kinase induces cyclooxygenase-2 and promotes chemoresistance through nuclear factor-kappaB pathway activation. Cancer Res. 2006;66(16):8123–8130. doi: 10.1158/0008-5472.CAN-06-1215. [DOI] [PubMed] [Google Scholar]
- Dorison A, Chantziantoniou C. DDR1: a major player in renal diseases. Cell Adh Migr. 2018;12(4):299–304. doi: 10.1080/19336918.2018.1441661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorison A, Dussaule JC, Chatziantoniou C. The role of discoidin domain receptor 1 in inflammation, fibrosis and renal disease. Nephron. 2017;137(3):212–220. doi: 10.1159/000479119. [DOI] [PubMed] [Google Scholar]
- Flamant M, Placier S, Rodenas A, Curat CA, Vogel WF, Chatziantoniou C, Dussaule JC. Discoidin domain receptor 1 null mice are protected against hypertension-induced renal disease. J Am Soc Nephrol. 2006;17(12):3374–3381. doi: 10.1681/ASN.2006060677. [DOI] [PubMed] [Google Scholar]
- Franco C, Britto K, Wong E, Hou G, Zhu SN, Chen M, Cybulsky MI, Bendeck MP. Discoidin domain receptor 1 on bone marrow-derived cells promotes macrophage accumulation during atherogenesis. Circ Res. 2009;105(11):1141–1148. doi: 10.1161/CIRCRESAHA.109.207357. [DOI] [PubMed] [Google Scholar]
- Gross O, Beirowski B, Harvey SJ, McFadden C, Chen D, Tam S, Thorner PS, Smyth N, Addicks K, Bloch W, Ninomiya Y, Sado Y, Weber M, Vogel WF. DDR1-deficient mice show localized subepithelial GBM thickening with focal loss of slit diaphragms and proteinuria. Kidney Int. 2004;66(1):102–111. doi: 10.1111/j.1523-1755.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- Guerrot D, Kerroch M, Placier S, Vandermeersch S, Trivin C, Mael-Ainin M, Chatziantoniou C, Dussaule JC. Discoidin domain receptor 1 is a major mediator of inflammation and fibrosis in obstructive nephropathy. Am J Pathol. 2011;179(1):83–91. doi: 10.1016/j.ajpath.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Greengard P, Nairn AC, Andersson T, Vogel WF. Phosphorylation of DARPP-32 regulates breast cancer cell migration downstream of the receptor tyrosine kinase DDR1. Exp Cell Res. 2006;312(20):4011–4018. doi: 10.1016/j.yexcr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry J, Scolyer RA, Davies MJ, Heinzelmann M, Kalish LH, Bali A, Kench JG, Edwards LS, Vanden Bergh PM, Hacker NF, Sutherland RL, O'Brien PM. Overexpression of the cell adhesion molecules DDR1, Claudin 3, and Ep-CAM in metaplastic ovarian epithelium and ovarian cancer. Clin Cancer Res. 2004;10(13):4427–4436. doi: 10.1158/1078-0432.CCR-04-0073. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2011;13(1):49–58. doi: 10.1038/ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G, Vogel W, Bendeck MP. The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest. 2001;107(6):727–735. doi: 10.1172/JCI10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Svoboda RA, Lazenby AJ, Saowapa J, Chaika N, Ding K, Wheelock MJ, Johnson KR. Up-regulation of N-cadherin by collagen i-activated discoidin domain receptor 1 in pancreatic cancer requires the adaptor molecule Shc1. J Biol Chem. 2016;291(44):23208–23223. doi: 10.1074/jbc.M116.740605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur H, Ham IH, Lee D, Jin H, Aguilera KY, Oh HJ, Han SU, Kwon JE, Kim YB, Ding K, Brekken RA. Discoidin domain receptor 1 activity drives an aggressive phenotype in gastric carcinoma. BMC Cancer. 2017;17(1):87. doi: 10.1186/s12885-017-3051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeitany M, Leroy C, Tosti P, Lafitte M, Le Guet J, Simon V, Bonenfant D, Robert B, Grillet F, Mollevi C, El Messaoudi S, Otandault A, Canterel-Thouennon L, Busson M, Thierry AR, Martineau P, Pannequin J, Roche S, Sirvent A. Inhibition of DDR1-BCR signalling by nilotinib as a new therapeutic strategy for metastatic colorectal cancer. EMBO Mol Med. 2018;10(4):e7918. doi: 10.15252/emmm.201707918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Ham IH, Oh HJ, Bae CA, Lee D, Kim YB, Son SY, Chwae YJ, Han SU, Brekken RA, Hur H. Inhibition of discoidin domain receptor 1 prevents stroma-induced peritoneal metastasis in gastric carcinoma. Mol Cancer Res. 2018;16(10):1590–1600. doi: 10.1158/1541-7786.MCR-17-0710. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Edman JC, Rutter WJ. A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain. Proc Natl Acad Sci U S A. 1993;90(12):5677–5681. doi: 10.1073/pnas.90.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Keerthikumar S, Raju R, Keshava Prasad TS, Ramachandra YL, Mohan S, Pandey A. PathBuilder–open source software for annotating and developing pathway resources. Bioinformatics. 2009;25(21):2860–2862. doi: 10.1093/bioinformatics/btp453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Mohan SS, Raju R, Keerthikumar S, Kumar GS, Venugopal AK, Telikicherla D, Navarro JD, Mathivanan S, Pecquet C, Gollapudi SK, Tattikota SG, Mohan S, Padhukasahasram H, Subbannayya Y, Goel R, Jacob HK, Zhong J, Sekhar R, Nanjappa V, Balakrishnan L, Subbaiah R, Ramachandra YL, Rahiman BA, Prasad TS, Lin JX, Houtman JC, Desiderio S, Renauld JC, Constantinescu SN, Ohara O, Hirano T, Kubo M, Singh S, Khatri P, Draghici S, Bader GD, Sander C, Leonard WJ, Pandey A. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11(1):R3. doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lee S, Suk K, Bark H, Jun CD, Kim DK, Choi CH, Yoshimura T. Discoidin domain receptor 1 mediates collagen-induced nitric oxide production in J774A.1 murine macrophages. Free Radic Biol Med. 2007;42(3):343–352. doi: 10.1016/j.freeradbiomed.2006.10.052. [DOI] [PubMed] [Google Scholar]
- Koo DH, McFadden C, Huang Y, Abdulhussein R, Friese-Hamim M, Vogel WF. Pinpointing phosphotyrosine-dependent interactions downstream of the collagen receptor DDR1. FEBS Lett. 2006;580(1):15–22. doi: 10.1016/j.febslet.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Kothiwale S, Borza CM, Lowe EW, Jr, Pozzi A, Meiler J. Discoidin domain receptor 1 (DDR1) kinase as target for structure-based drug discovery. Drug Discov Today. 2015;20(2):255–261. doi: 10.1016/j.drudis.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutmon M, van Iersel MP, Bohler A, Kelder T, Nunes N, Pico AR, Evelo CT. PathVisio 3: an extendable pathway analysis toolbox. PLoS Comput Biol. 2015;11(2):e1004085. doi: 10.1371/journal.pcbi.1004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaguarnera R, Nicolosi ML, Sacco A, Morcavallo A, Vella V, Voci C, Spatuzza M, Xu SQ, Iozzo RV, Vigneri R, Morrione A, Belfiore A. Novel cross talk between IGF-IR and DDR1 regulates IGF-IR trafficking, signaling and biological responses. Oncotarget. 2015;6(18):16084–16105. doi: 10.18632/oncotarget.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata R, Palladino C, Nicolosi ML, Lo Presti AR, Malaguarnera R, Ragusa M, Sciortino D, Morrione A, Maggiolini M, Vella V, Belfiore A. IGF-I induces upregulation of DDR1 collagen receptor in breast cancer cells by suppressing MIR-199a-5p through the PI3K/AKT pathway. Oncotarget. 2016;7(7):7683–7700. doi: 10.18632/oncotarget.6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matada GSP, Das A, Dhiwar PS, Ghara A. DDR1 and DDR2: a review on signaling pathway and small molecule inhibitors as an anticancer agent. J Med Chem Res. 2021;30(3):535–551. doi: 10.1007/s00044-020-02694-2. [DOI] [Google Scholar]
- Matsuyama W, Wang L, Farrar WL, Faure M, Yoshimura T. Activation of discoidin domain receptor 1 isoform b with collagen up-regulates chemokine production in human macrophages: role of p38 mitogen-activated protein kinase and NF-kappa B. J Immunol. 2004;172(4):2332–2340. doi: 10.4049/jimmunol.172.4.2332. [DOI] [PubMed] [Google Scholar]
- Miao L, Zhu S, Wang Y, Li Y, Ding J, Dai J, Cai H, Zhang D, Song Y. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung cancer and promotes cell invasion via epithelial-to-mesenchymal transition. Med Oncol. 2013;30(3):626. doi: 10.1007/s12032-013-0626-4. [DOI] [PubMed] [Google Scholar]
- Nakada M, Kita D, Teng L, Pyko IV, Watanabe T, Hayashi Y, Hamada J. Receptor tyrosine kinases: principles and functions in glioma invasion. Adv Exp Med Biol. 2013;986:143–170. doi: 10.1007/978-94-007-4719-7_8. [DOI] [PubMed] [Google Scholar]
- Nemoto T, Ohashi K, Akashi T, Johnson JD, Hirokawa K. Overexpression of protein tyrosine kinases in human esophageal cancer. Pathobiology. 1997;65(4):195–203. doi: 10.1159/000164123. [DOI] [PubMed] [Google Scholar]
- Park HS, Kim KR, Lee HJ, Choi HN, Kim DK, Kim BT, Moon WS. Overexpression of discoidin domain receptor 1 increases the migration and invasion of hepatocellular carcinoma cells in association with matrix metalloproteinase. Oncol Rep. 2007;18(6):1435–1441. [PubMed] [Google Scholar]
- Quan J, Yahata T, Adachi S, Yoshihara K, Tanaka K. Identification of receptor tyrosine kinase, discoidin domain receptor 1 (DDR1), as a potential biomarker for serous ovarian cancer. Int J Mol Sci. 2011;12(2):971–982. doi: 10.3390/ijms12020971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romayor I, Badiola I, Olaso E. Inhibition of DDR1 reduces invasive features of human A375 melanoma, HT29 colon carcinoma and SK-HEP hepatoma cells. Cell Adh Migr. 2020;14(1):69–81. doi: 10.1080/19336918.2020.1733892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo MC, Kim S, Kim SH, Zheng LT, Park EK, Lee WH, Suk K. Discoidin domain receptor 1 mediates collagen-induced inflammatory activation of microglia in culture. J Neurosci Res. 2008;86(5):1087–1095. doi: 10.1002/jnr.21552. [DOI] [PubMed] [Google Scholar]
- Shen Q, Cicinnati VR, Zhang X, Iacob S, Weber F, Sotiropoulos GC, Radtke A, Lu M, Paul A, Gerken G, Beckebaum S. Role of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion. Mol Cancer. 2010;9:227. doi: 10.1186/1476-4598-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Nakamura M, Ishida E, Higuchi T, Yamamoto H, Tsujikawa K, Konishi N. Prostate cancer antigen-1 contributes to cell survival and invasion though discoidin receptor 1 in human prostate cancer. Cancer Sci. 2008;99(1):39–45. doi: 10.1111/j.1349-7006.2007.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y, Fukumoto Y, Chaika N, Svoboda R, Wheelock MJ, Johnson KR. Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol. 2008;180(6):1277–1289. doi: 10.1083/jcb.200708137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1(1):25–34. doi: 10.1016/S1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- Song J, Chen X, Bai J, Liu Q, Li H, Xie J, Jing H, Zheng J. Discoidin domain receptor 1 (DDR1), a promising biomarker, induces epithelial to mesenchymal transition in renal cancer cells. Tumour Biol. 2016;37(8):11509–11521. doi: 10.1007/s13277-016-5021-2. [DOI] [PubMed] [Google Scholar]
- Tao Y, Wang R, Lai Q, Wu M, Wang Y, Jiang X, Zeng L, Zhou S, Li Z, Yang T, Yao Y, Wu Y, Yu L, Fu Y, Lai W, Peng Y, Lu Y, Zhang Z, Guo C, Zhang G, Gou L, Yang J. Targeting of DDR1 with antibody-drug conjugates has antitumor effects in a mouse model of colon carcinoma. Mol Oncol. 2019;13(9):1855–1873. doi: 10.1002/1878-0261.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy KA, Valiathan RR, Nunez F, Kidwell KM, Gonzalez ME, Fridman R, Kleer CG. Tyrosine kinase discoidin domain receptors DDR1 and DDR2 are coordinately deregulated in triple-negative breast cancer. Breast Cancer Res Treat. 2015;150(1):9–18. doi: 10.1007/s10549-015-3285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 2012;31(1–2):295–321. doi: 10.1007/s10555-012-9346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehlow A, Klapproth E, Jin S, Hannen R, Hauswald M, Bartsch JW, Nimsky C, Temme A, Leitinger B, Cordes N. Interaction of discoidin domain receptor 1 with a 14–3–3-Beclin-1-Akt1 complex modulates glioblastoma therapy sensitivity. Cell Rep. 2019;26(13):3672–3683. doi: 10.1016/j.celrep.2019.02.096. [DOI] [PubMed] [Google Scholar]
- Vella V, Malaguarnera R, Nicolosi ML, Palladino C, Spoleti C, Massimino M, Vigneri P, Purrello M, Ragusa M, Morrione A, Belfiore A. Discoidin domain receptor 1 modulates insulin receptor signaling and biological responses in breast cancer cells. Oncotarget. 2017;8(26):43248–43270. doi: 10.18632/oncotarget.18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella V, Nicolosi ML, Cantafio P, Massimino M, Lappano R, Vigneri P, Ciuni R, Gangemi P, Morrione A, Malaguarnera R, Belfiore A. DDR1 regulates thyroid cancer cell differentiation via IGF-2/IR-A autocrine signaling loop. Endocr Relat Cancer. 2019;26(1):197–214. doi: 10.1530/ERC-18-0310. [DOI] [PubMed] [Google Scholar]
- Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1(1):13–23. doi: 10.1016/S1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- Vogel WF, Abdulhussein R, Ford CE. Sensing extracellular matrix: an update on discoidin domain receptor function. Cell Signal. 2006;18(8):1108–1116. doi: 10.1016/j.cellsig.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Weiner HL, Huang H, Zagzag D, Boyce H, Lichtenbaum R, Ziff EB. Consistent and selective expression of the discoidin domain receptor-1 tyrosine kinase in human brain tumors. Neurosurgery. 2000;47(6):1400–1409. doi: 10.1097/00006123-200012000-00028. [DOI] [PubMed] [Google Scholar]
- Willenbrock K, Kuppers R, Renne C, Brune V, Eckerle S, Weidmann E, Brauninger A, Hansmann ML. Common features and differences in the transcriptome of large cell anaplastic lymphoma and classical Hodgkin's lymphoma. Haematologica. 2006;91(5):596–604. [PubMed] [Google Scholar]
- Xie X, He H, Zhang N, Wang X, Rui W, Xu D, Zhu Y. Overexpression of DDR1 promotes migration, invasion, though EMT-related molecule expression and COL4A1/DDR1/MMP-2 signaling axis. Technol Cancer Res Treat. 2020;19:1533033820973277. doi: 10.1177/1533033820973277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka R, Arao T, Yajima N, Tsuchiya N, Homma J, Tanaka R, Sano M, Oide A, Sekijima M, Nishio K. Identification of expressed genes characterizing long-term survival in malignant glioma patients. Oncogene. 2006;25(44):5994–6002. doi: 10.1038/sj.onc.1209585. [DOI] [PubMed] [Google Scholar]
- Yang SH, Baek HA, Lee HJ, Park HS, Jang KY, Kang MJ, Lee DG, Lee YC, Moon WS, Chung MJ. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung carcinomas. Oncol Rep. 2010;24(2):311–319. doi: 10.3892/or_00000861. [DOI] [PubMed] [Google Scholar]
- Ye L, Pu C, Tang J, Wang Y, Wang C, Qiu Z, Xiang T, Zhang Y, Peng W. Transmembrane-4 L-six family member-1 (TM4SF1) promotes non-small cell lung cancer proliferation, invasion and chemo-resistance through regulating the DDR1/Akt/ERK-mTOR axis. Respir Res. 2019;20(1):106. doi: 10.1186/s12931-019-1071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida D, Teramoto A. Enhancement of pituitary adenoma cell invasion and adhesion is mediated by discoidin domain receptor-1. J Neurooncol. 2007;82(1):29–40. doi: 10.1007/s11060-006-9246-6. [DOI] [PubMed] [Google Scholar]
- Yoshida D, Teramoto A. The use of 3-D culture in peptide hydrogel for analysis of discoidin domain receptor 1-collagen interaction. Cell Adh Migr. 2007;1(2):92–98. doi: 10.4161/cam.1.2.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zhao S, Luo L, Xiang Q, Zhu Z, Wang J, Liu Y, Luo J. miR-199b-5p-DDR1-ERK signalling axis suppresses prostate cancer metastasis via inhibiting epithelial-mesenchymal transition. Br J Cancer. 2021;124(5):982–994. doi: 10.1038/s41416-020-01187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.