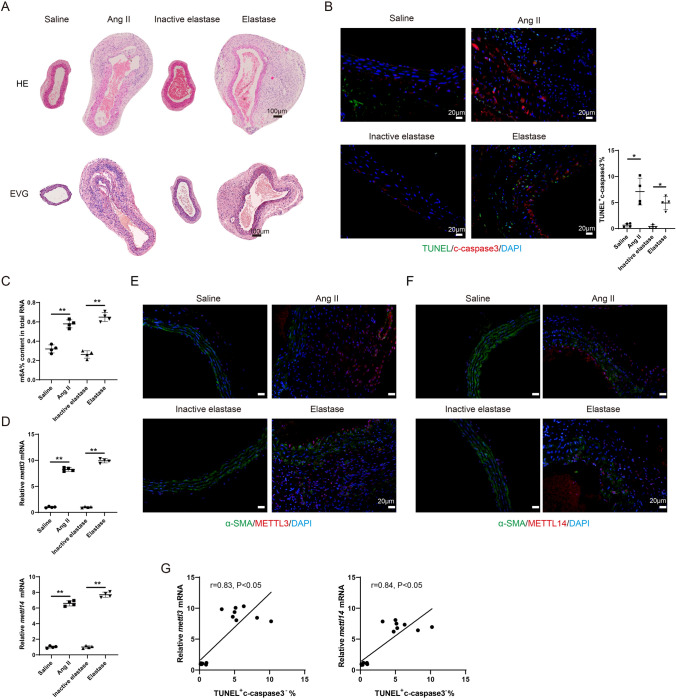
Fig. 1.
The level of m6A modification is elevated during the AAA pathological process. The mouse AAA model was induced with Angiotensin II (Ang II) or Elastase. ApoE − / − mice were subcutaneously infused with Ang II via an implanted osmotic minipump (the Ang II group, n = 4). The control ApoE − / − mice were subcutaneously infused with the same volume of normal saline (the Saline group, n = 4). The infusion rate was 1000 ng/kg/min and the infusion lasted for 28 days. Type I pancreatic porcine elastase (6 IU/mg) was locally applied on C57BL/6 mice for 5 min (the Elastase group, n = 4). An equal concentration of heat-inactivated (100 °C for 15 min) elastase was used as the control (the Inactive elastase group, n = 4). A HE staining and the EVG staining of the mouse aortic tissues. B Immunofluorescence staining of the mouse aortic tissues using anti-c-caspase 3 and TUNEL assay (Brown-Forsythe and Welch ANOVA test with Games-Howell’s post-hoc test). C The m6A% content in total RNA in abdominal aortic wall tissues was detected using the EpiQuik m6A RNA Methylation Quantification Kit (**P < 0.01, one-way ANOVA with Tukey’s post-hoc test). D Relative mRNA expressions of mettl3 and mettl14 in mouse aortic tissues were detected using the qRT-PCR. gapdh was used as the internal control. Relative gene expression was calculated using the 2−△△Ct method (**P < 0.01, one-way ANOVA with Tukey’s post-hoc test). E–F Immunofluorescence staining of the mouse aortic tissues using anti-α-SMA, anti-METTL3, and anti-METTL14 (Scale Bar = 20 μm). G The relationship between the expression of mettl3/mettl14 and the percentage of TUNEL+ c-caspase 3− cells were determined using Pearson’s correlation analysis (n = 16, Pearson r test)