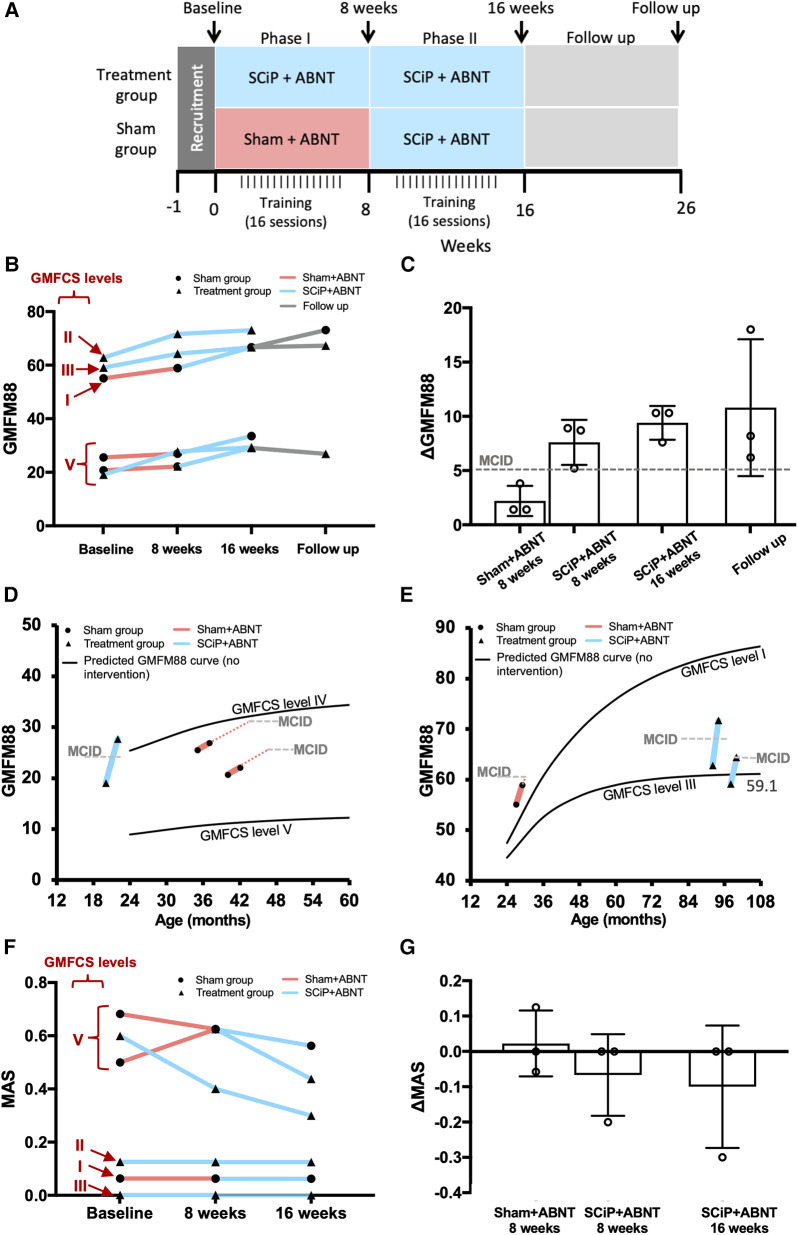
Figure 1.
(A) Experimental design and timeline of events. (B) GMFM88 scores at baseline, week 8, week 16 and follow up. 3 participants started with SCiP™ + ABNT (blue) vs. 3 with sham + ABNT (red). Sham group crossed over to SCiP™ at week 8 and all participants received SCiPTM from week 8 to 16, followed by 10 weeks of no intervention (grey). (C) Mean ± SD change from baseline in GMFM88 scores after 8 weeks of sham, 8 weeks of SCiP™, 16 weeks of SCiP™ and follow up (n = 3 each). (D,E) Comparison of GMFM88 scores at the end of 8 weeks (primary efficacy endpoint) between sham (red) and SCiP™ (blue) groups with reference to a validated predicted model of change in GMFM scores without an intervention, matched for age and GMFCS level (21). (F) spasticity scores (MAS) at baseline, week 8 and week 16 for sham (red) and SCiP™ (blue) groups. (G) Mean ± SD change in spasticity scores suggest that children in therapeutic group had lower spasticity at the end of 8 and 16 weeks, compared to baseline.