Figure 3. Secondary, Exploratory and Subgroup Efficacy Measures from Part 2.
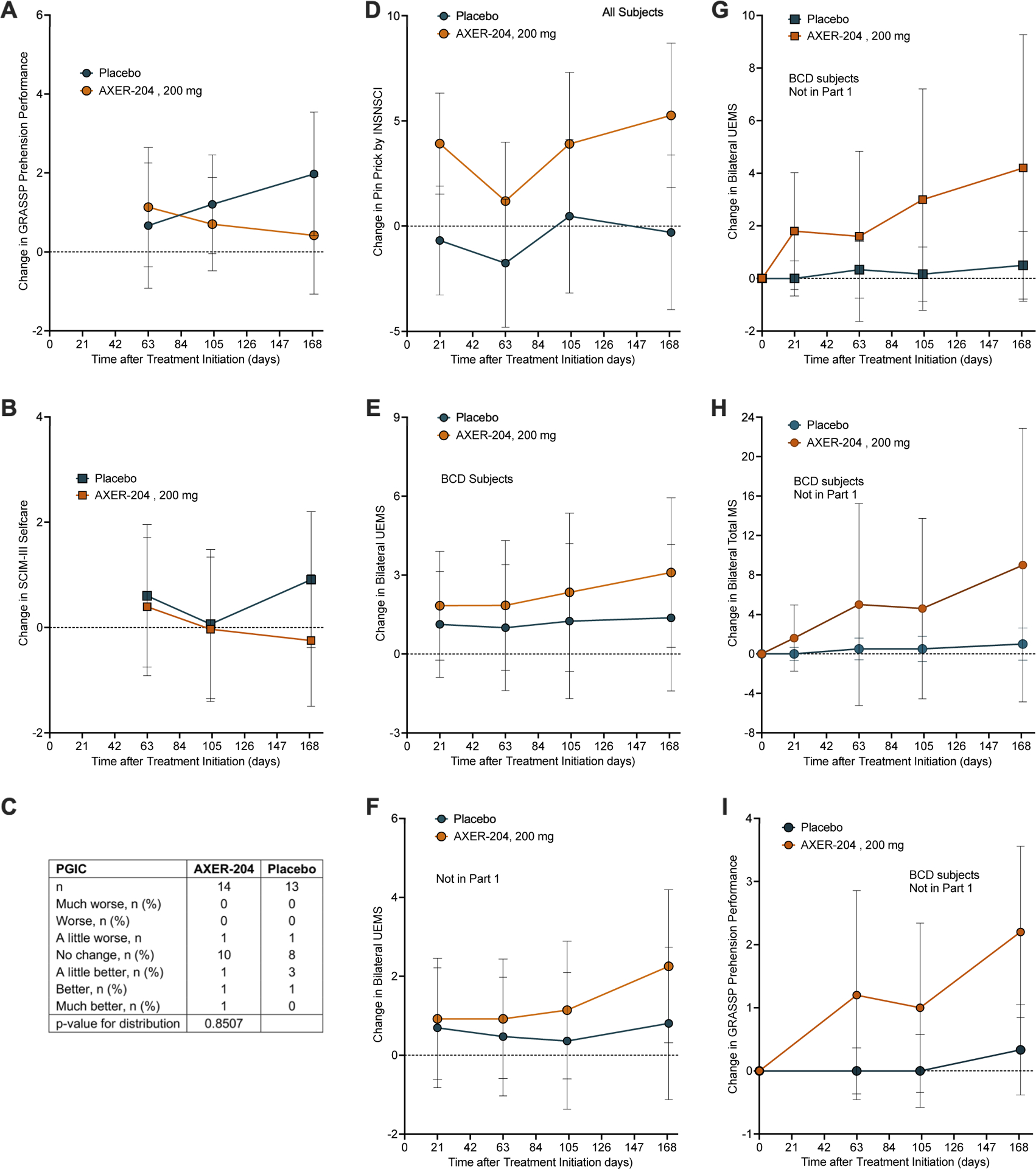
(A) Change from baseline in bilateral GRASSP Prehension Performance score by mixed-effects model for repeated measures (MMRM) analysis for part 2 groups. Figure shows least squares mean ± 95% CI for n =14 for AXER-204 and n=13 for placebo. By MRMM, non-significant.
(B) Change from baseline in SCIM III self-care by mixed-effects model for repeated measures (MMRM) analysis for part 2 groups. Figure shows least squares mean ± 95% CI for n =14 for AXER-204 and n=13 for placebo. By MRMM, non-significant.
(C) Patient Global Impression of Change (PGIC) from baseline to Day 169 for part 2 groups.
(D) Change from baseline in bilateral Pin Prick sensory score of the ISNCSCI examination by mixed-effects model for repeated measures (MMRM) analysis for part 2 groups is plotted. The least squares mean ± 95% CI is plotted for n =14 for AXER-204 and n=13 for placebo. By MRMM, non-significant.
(E) Preplanned subgroup analysis for BCD participants of change in bilateral UEMS from the ISNCSCI examination. The least squares mean ± 95% CI is plotted for n=8 for AXER-204 and n=8 for placebo. By MRMM, non-significant.
(F) Preplanned subgroup analysis for participants not enrolled in part 1 of change in bilateral UEMS from the ISNCSCI examination. The least squares mean ± 95% CI is plotted for n=9 for AXER-204 and n=9 for placebo. By MRMM, non-significant.
(G) For BCD participants not enrolled in part 1, a post hoc analysis of change from baseline in bilateral UEMS from ISNCSCI examination. Data are arithmetic mean ± 95% CI for n=6 for AXER-204 and n=5 for placebo. By repeated measures ANOVA, time*group (P = 0.026), time (P = 0.032), and group (P = 0.072).
(H) For BCD participants not enrolled in part 1, a post hoc analysis of change from baseline in total MS from ISNCSCI examination. Data are arithmetic mean ± 95% CI for n=6 for AXER-204 and n=5 for placebo. By repeated measures ANOVA, non-significant.
(I) For BCD participants not enrolled in part 1, a post hoc analysis of change from baseline in GRASSP Prehension Performance and total MS of the ISNCSCI examination. Data are arithmetic mean ± 95% CI for n=6 for AXER-204 and n=5 for placebo. By repeated measures ANOVA, non-significant.
