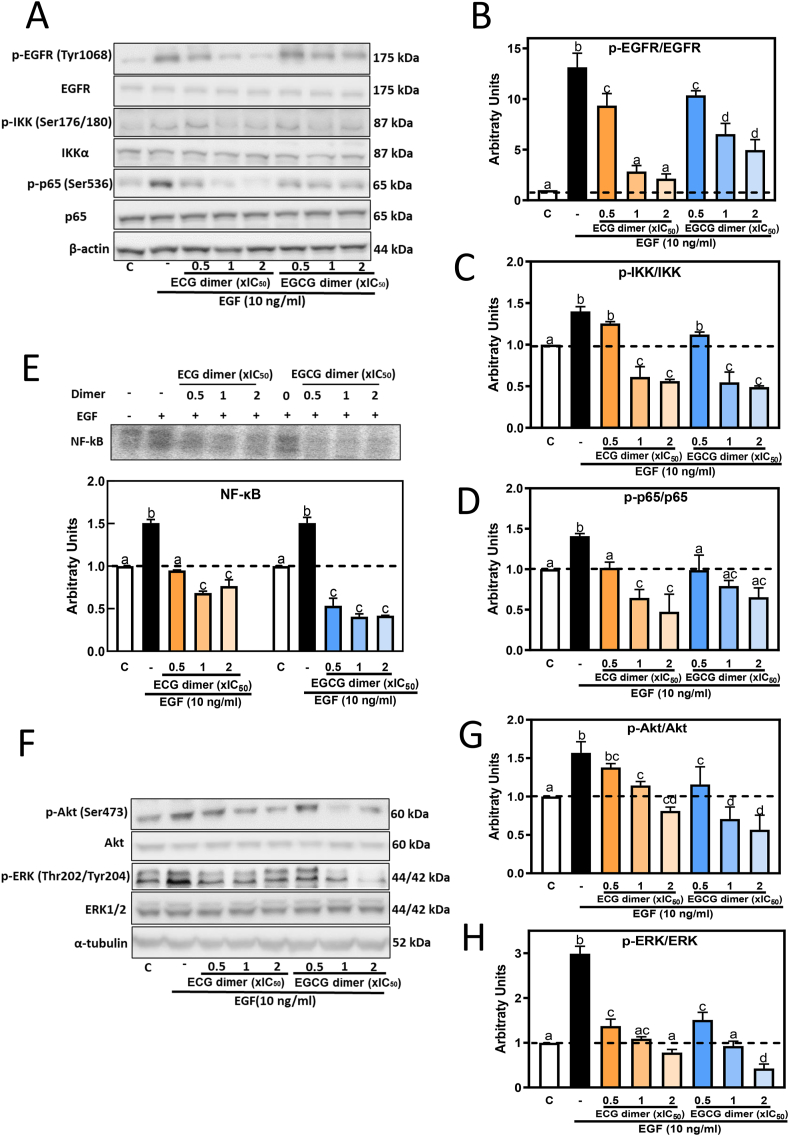
Fig. 4.
ECG and EGCG dimers inhibited EGF-mediated activation of the EGFR and downstream of NF-κB, Akt, and ERK1/2 pathways. Caco-2 cells were pre-incubated with or without ECG and EGCG dimers for 30 min and then with or without EGF (10 ng/ml) for subsequent 10 min. Phosphorylation levels of B- EGFR (Tyr1068), C– IKK (Ser176/180), D- p65 (Ser536), G- Akt (Ser473), and H- ERK1/2 ((Thr202/Tyr204) were evaluated by Western blot. A, F- Representative Western blot images. Bands were quantified and values for phosphorylated proteins were referred to the respective total protein content. E− NF-κB activation was also evaluated by EMSA measuring NF-κB-DNA binding in nuclear fractions. Results are shown as means ± SEM of 3–5 independent experiments. Values having different superscripts are significantly different (p < 0.05, One-way ANOVA-test).