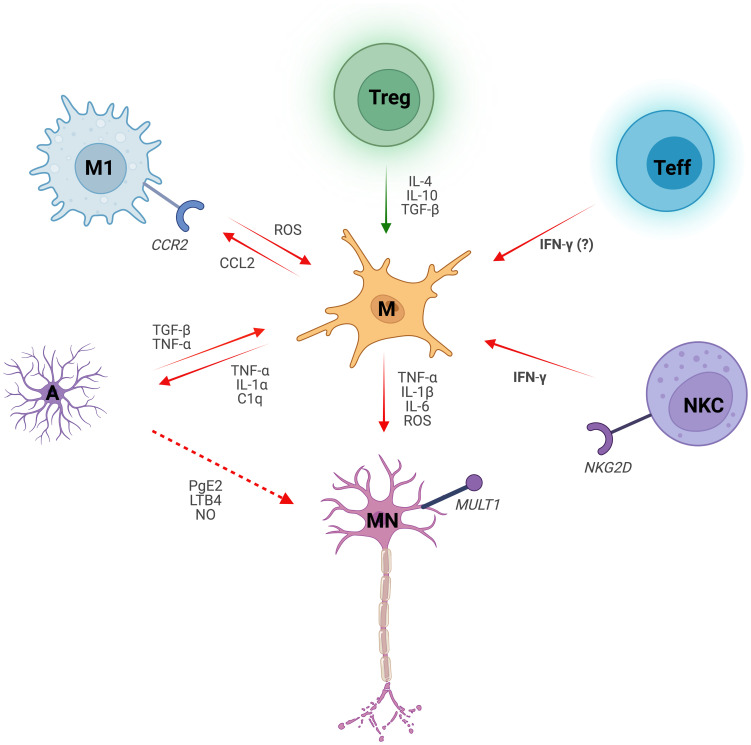
Figure 1.
Microglial dialogue with non-neuronal cells in Amyotrophic Lateral Sclerosis. Microglia (M) induce motor neuron (MN) degeneration in ALS by secreting reactive oxygen species (ROS) and pro-inflammatory cytokines, such as Interleukin 1 beta (IL-1β), Interleukin 6 (IL-6) and Tumor Necrosis Factor (TNF-α). Microglial crosstalk with non-neuronal cells shapes their phenotype, either skewing it towards a pro-inflammatory (red arrows) on anti-inflammatory (green arrows) phenotype. Microglial-derived pro-inflammatory cytokines Interleukin 1 alpha (IL-1α), TNFα and complement component C1q induce pro-inflammatory astrocytes (A). Conversely, activated astrocytes promote inflammatory microglial responses via Transforming Growth Factor β (TGF-β) and TNF‐α. Reactive astrocytes also exert toxic effects on MNs by secreting inflammatory mediators such as Prostaglandin E2 (PgE2), Leukotriene B4 (LBT4) and nitric oxide (NO). Chemokine ligand 2 receptor (CCR2)-expressing macrophages (M1) are recruited by the Chemokine Ligand 2 (CCL2) released by microglia. ROS pathway in classically activated macrophages induces microglial activation. Regulatory T cells (Treg) suppress microglial toxicity as well as other immune cells (not shown) through Interleukin 4 (IL-4), Interleukin 10 (IL-10) and TGF-β. Notably, TGF-β effect on microglia is context- and cell-dependent. Microglia-CD8+ Effector T cell (Teff) crosstalk drives neuroinflammation in ALS, with Interferon gamma (IFN-γ) secreted by the latter likely playing a role. Infiltrated Natural Killer Cells (NKC) instruct microglia towards an inflammatory profile by the release of IFN-γ. Additionally, NKCs are neurotoxic to MNs via NKG2D - NKG2D ligand (MULT1) interaction.