Abstract
Objective:
Primary progressive apraxia of speech (PPAOS) is associated with imaging abnormalities in lateral premotor cortex (LPC) and supplementary motor area (SMA). It’s unknown whether relatively greater involvement of these regions in either hemisphere is associated with demographics, presenting, and/or longitudinal features.
Methods:
In 51 prospectively recruited PPAOS patients who completed [18F]-fluorodeoxyglucose (FDG) PET we classified patients as left-dominant, right-dominant, or symmetric, based on visual assessment of the LPC and SMA on FDG-PET. SPM and statistical analysis of regional metabolic values were performed. Diagnosis of PPAOS was made if AOS was present and aphasia absent. Thirteen patients completed Ioflupane-123I (DAT) scans. We compared cross-sectional and longitudinal clinicopathological, genetic, and neuroimaging characteristics across the three groups, with area under the receiver operator curve (AUROC) determined as a measure of effect size.
Results:
49% of the PPAOS patients were classified as left-dominant, 31% as right-dominant, and 20% as symmetric which was supported by results from the SPM and regional analyses. There were no differences in baseline characteristics. Longitudinally, right-dominant PPAOS showed faster rates of progression of ideomotor apraxia (AUROC=0.79), behavioral disturbances (AUROC=0.84), including disinhibition symptoms (AUROC=0.82) and negative behaviors (AUROC=0.82), and Parkinsonism (AUROC=0.75), compared to left-dominant PPAOS. Symmetric PPAOS showed faster rates of dysarthria progression compared to left-dominant (AUROC=0.89) and right-dominant PPAOS (AUROC=0.79). Five patients showed abnormal DAT uptake. Braak neurofibrillary tangle stage differed across groups (p=0.01).
Conclusions:
Patients with PPAOS and a right-dominant pattern of hypometabolism on FDG-PET have fastest rates of decline of behavioral and motor features.
Keywords: PPAOS, Supplementary motor area, Premotor, FDG-PET, DaTscan
INTRODUCTION
Apraxia of Speech (AOS) is a neurological speech disorder characterized by impairments in the ability to plan and/or program movements involved in speech production [1] [2] [3] [4] [5]. Apraxia of speech often co-exists with aphasia and/or dysarthria but there are frequent instances when AOS is the sole presenting symptom. When AOS is the sole presenting symptom, a diagnosis of primary progressive apraxia of speech (PPAOS) is rendered [2]. Hence, the paramount clinical feature of PPAOS is a progressive isolated speech production disorder from impaired programming and planning of speech[3], although aphasia and dysarthria can develop over time. In PPAOS, patients often demonstrate a predominance of phonetic or prosodic speech abnormalities, thus establishing the presence of two distinctive subtypes; a phonetic subtype and the prosodic subtype [6] [7]. If neither phonetic nor prosodic features predominate, patients are classified as mixed [6, 8]. It should be noted that PPAOS is considered distinct, by some investigators, from the non-fluent/agrammatic variant of primary progressive aphasia (nfvPPA) where language deficit, specifically grammatical impairment, is the salient feature of the presenting syndrome [9] [10] [11, 12].
Neuroimaging abnormalities occur in PPAOS affecting the lateral premotor cortex (LPC) and the supplementary motor area (SMA) and while such abnormalities are often subtle on MRI, abnormalities are more easily observed on [18F] fluorodeoxyglucose (FDG) PET [13] [14] [15] [14]. Some patients display left LPC/SMA dominance on FDG-PET, others right LPC/SMA dominance, and others equally prominent involvement of the LPC and SMA in both hemispheres. There are no studies that have assessed whether clinical differences associate with which hemisphere is more affected. Clinically relevant differences between groups may be influential to patient care and prognosis.
To address this knowledge gap, the current study aimed to determine whether there are differences in demographics, clinical, neuroimaging, pathological, or genetic features, between left-dominant, right-dominant, and symmetric PPAOS. We hypothesized that left-dominant PPAOS would develop aphasia at a faster rate compared to right-dominant PPAOS, due to the left hemisphere’s involvement in language functions.
METHODS
Study Design and Participants
We conducted a case-control study of 51 participants with PPAOS. All participants had been recruited and prospectively followed by the Neurodegenerative Research Group (NRG), Mayo Clinic, Rochester MN, and enlisted into NIH-funded grants between 7/1/2020 and 6/30/2022. At initial research visits, all participants completed an FDG-PET scan and a battery of speech and language, and neuropsychological testing measures, as previously reported [2] [16] [17]. All participants also underwent MRI and Pittsburgh Compound-B (PiB) PET to assess beta-amyloid deposition. Thirteen had completed an Ioflupane 123I scan (Dopamine Transporter, DAT, scan, GE Healthcare, Chicago, IL). Thirty-nine participants (76%) underwent yearly longitudinal follow-ups, with identical clinical assessments at each visit. All participants gave a blood sample to allow for apolipoprotein-E (APOE) genotyping. Of the 51 participants, 35 have died with 23 undergoing a brain autopsy.
Speech and Language Evaluation
All speech and language assessments were carried out by one of three experienced speech-language pathologists (J.R.D, H.M.C., R.L.U). Speech and language evaluations were audio and video recorded. Two or more speech-language pathologists reviewed all audio/video recordings at a consensus meeting whereby a consensus diagnosis was made. In the absence of consensus, the third speech-language pathologist served as the tiebreaker. The Western Aphasia Battery aphasia quotient (WAB-AQ) [18] was utilized to measure global language ability and aphasia severity with the Northwestern Anagram Test (NAT) [19] used to assess syntactic comprehension. The AOS rating scale (ASRS) was used to assess AOS severity [20] and the Motor Speech Disorder severity (MSD) scale (adapted from [21]) was used to rate the degree to which speech is understandable (i.e., functional impairment). To be diagnosed with PPAOS, participants had to have evidence of AOS with no more than equivocal evidence of agrammatism [2]. Patients were excluded from the study if they displayed more than equivocal evidence of aphasia during speech and language testing. AOS subtype was based on AOS characteristics judged to be dominant as previously described [6, 8]. Dysarthria severity was also rated on a 0 to 4 scale [2].
Neurological Evaluations
Neurologic examinations were performed by an experienced, board-certified behavioral neurologist at the time of enrollment and follow-up (K.A.J., H. B.). The examinations included the Montreal Cognitive Assessment (MoCA) [22], the WAB Limb Apraxia subtest to assess ideomotor apraxia, the Movement Disorders Society sponsored revision of the Unified Parkinson’s Disease Rating Scale part III [23] to assess parkinsonism, the Neuropsychiatric Inventory Questionnaire (NPI-Q) [24] to assess neuropsychiatric features, the Frontal Behavioral Inventory (FBI) to assess abnormal behaviors, and the FBI subtests for Disinhibition and Negative Behaviors [25].
Neuroimaging
Image acquisition for FDG-PET
All PET scans were acquired using a GE PET/CT scanner. Participants were injected with FDG (average, 459 MBq; range, 367–576 MBq) and following a 30-minute uptake period, an 8-minute scan was administered utilizing four 2-minute dynamic frames [2] [26]. Participants were injected with PiB of approximately 628 MBq (range, 385–723 MBq) and after a 40-to-60-minute uptake period, a 20-minute scan was obtained. Standard corrections were adjusted for and data were reconstructed into a 256×256 matrix with a 30-cm field of view (image thickness=3.75 mm) [2]. If motion was detected single frames of the PET were realigned and a mean image created [16]. A global PiB standard uptake value ratio (SUVR) was calculated as previously described [27], with a cut-point of ≥1.48 used to determine beta-amyloid positivity. All participants also underwent a volumetric head MRI within one day of the FDG-PET that included a magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence.
Group designation based on FDG-PET
Individual-level patterns of hypometabolism on the baseline FDG-PET scans were analyzed using 3D stereotactic surface projections using CortexID suite (GE Healthcare) [28] whereby activity at each voxel is normalized to the pons and Z-scored to an age-segmented normative database. The Cortex ID FDG-PET images underwent independent visual analysis by a neurology (K.A.J.) and radiology expert (J.L.W.) blinded to all clinical/pathological/genetic data to classify participants as left-dominant, right-dominant or symmetric PPAOS. A diagnosis of left-dominant PPAOS was made if the left LPC and SMA showed greater hypometabolism than the corresponding right LPC and SMA. A diagnosis of right-dominant PPAOS was made if the right LPC and SMA showed greater hypometabolism than the corresponding left LPC and SMA. Cases in which there was approximately equal hypometabolism in right and left LPC and SMA were categorized as symmetric PPAOS. In cases where there was disagreement (n=2) both classifiers came to a consensus after further discussion. Representative FDG-PET images of left-dominant, right-dominant, and symmetric PPAOS participants are shown in Figure 1.
Figure 1:
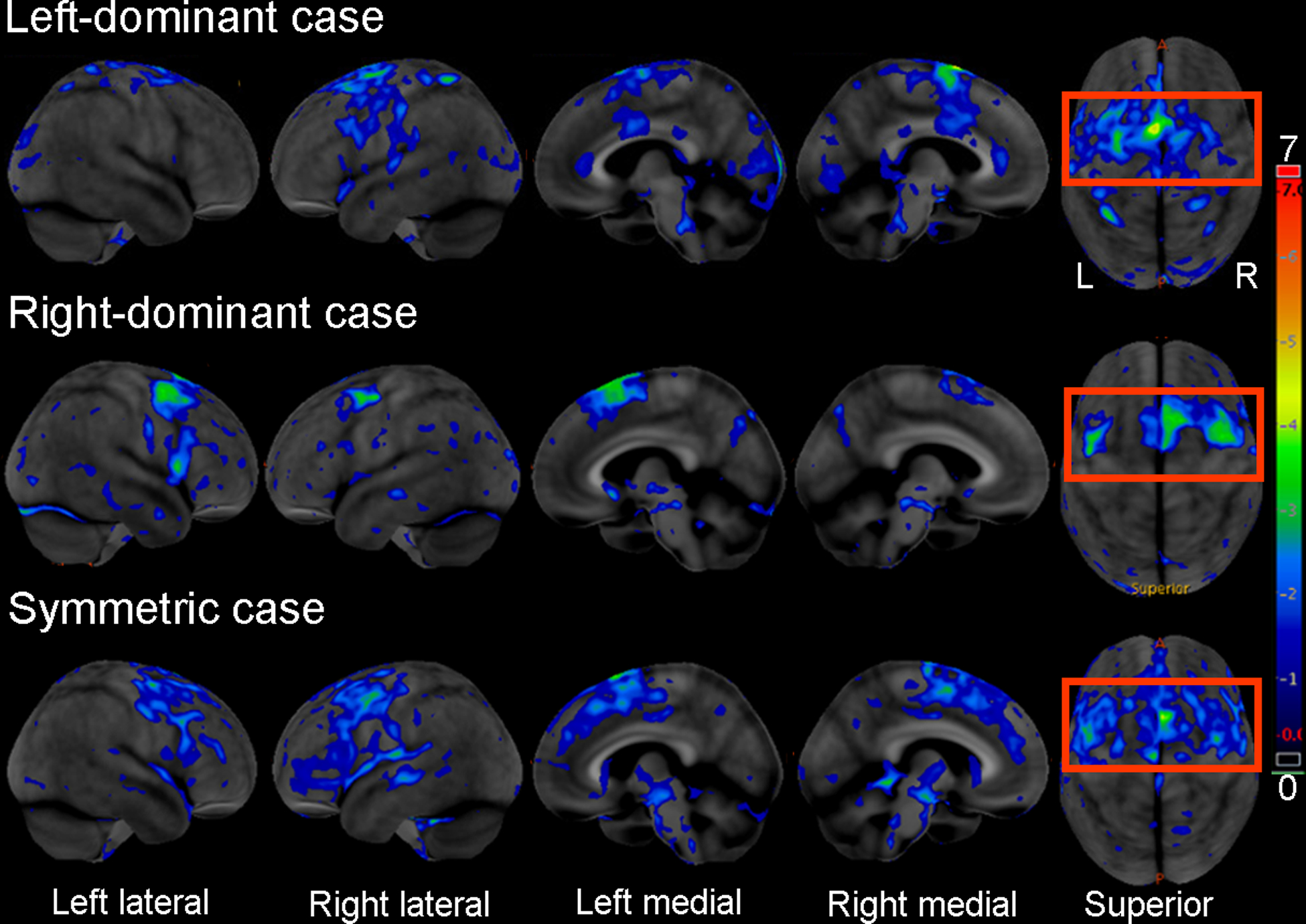
[18F]-fluorodeoxyglucose (FDG)-positron emission tomography (PET) across examples of left dominant, right dominant and symmetric PPAOS patients. The 3 panels depict Z scores relative to a normative database of pons intensity normalized FDG-PET scans for each individual displayed on stereotactic surface projections using Cortex ID. Red coloring indicates greater hypometabolism or decreased brain glucose consumption.
SPM analysis of FDG-PET
To evaluate group-level patterns of hypometabolism across the brain, voxel-level analyses of FDG-PET were performed in SPM12. Each FDG-PET was registered to the participant’s MPRAGE using 6 degrees-of-freedom registration. MPRAGE scans were normalized to the Mayo Clinic Adult Lifespan Template (MCALT). All voxels in the MPRAGE space FDG-PET images were divided by median uptake in the pons employing the MCALT atlas to create standardized uptake value ratio (SUVR) images. The SUVR images were transformed to MCALT space and smoothed at 6mm full-width-at-half-maximum. Analyses were performed without partial volume correction (PVC). The left-dominant, right-dominant, and symmetric PPAOS groups were compared to 20 cognitively and motorically intact healthy controls (11 females/9 males, average [range] age=71.3 [61.0, 86.2], MoCA=26.4 [24, 30], Hoehn and Yahr Scale=0 [0, 0]), with comparisons performed using family-wise error (FWE) correction at p<0.05.
Semi-quantitative and quantitative measures of hypometabolism of premotor cortex and Broca’s area
The degree of hypometabolism in the premotor cortex (LPC and SMA) and Broca’s area were visually graded on CORTEX-ID images using a 5-point scale: 0= no hypometabolism, 1= minimal hypometabolism, 2= mild hypometabolism, 3= moderate hypometabolism and 4 = severe hypometabolism, by two independent raters (KAJ and JLW). Individual ratings were then averaged across both raters and compared across the 3 groups (left-dominant, right-dominant, and symmetric PPAOS). Quantitative measures of metabolism were also calculated for the left and right SMA and Broca’s area (combined inferior frontal triangularis and inferior frontal opercularis) using the MCALT atlas. All regional uptake values were divided by uptake in the pons to create SUVRs.
DAT scan analysis
DAT scans were acquired using a 5 mCi (±10%) dose in a GE Tandem Optima SPECT scanner equipped with a fan-beam collimator (GE Healthcare, Chicago, IL). After images were acquired, they were then reconstructed through the ordered subset expectation maximization (OSEM) method. No attenuation correction was used. A nuclear medicine specialist (VL) independently visually rated the scans as being normal or abnormal, including hemispheric asymmetry.
Pathologic Evaluation
All participants who died and agreed to autopsy underwent brain autopsy by a board-certified neuropathologist (D.W.D) according to the Consortium to Establish a Registry for Alzheimer’s Disease [29]. Thioflavin S fluorescent microscopy was used to assign Braak neurofibrillary tangle (NFT) stage [30] and Thal beta-amyloid phase [31]. Pathological diagnoses were rendered based on published criteria [32–35].
Statistical Analysis
Non-parametric tests included Kruskal-Wallis tests to compare continuous variables, while Fisher’s Exact test was used to compare categorical variables. For all longitudinal data, worsening overtime was calculated using the average rate of change (points change on each scale per year) from testing at the time of a participants first FDG-PET scan and at their last visit. Two group comparisons correcting for multiple comparisons were performed using Dunn’s multiple comparisons test. To determine effect sizes to differentiate between groups we calculated the area under the receiver operator curve (AUROC) via the trapezoid rule and nonlinear regression. All statistical analyses were performed using Prism 8 (version 9.2; GraphPad Software Inc) and R studio (version 4.12).
RESULTS
Demographic, genetic, and pathology results
Demographic data are summarized in Table 1 and Figure 2. Of the 51 PPAOS participants, 25 (49%) were classified as having left-dominant hypometabolism on FDG-PET, 16 (31%) as right-dominant, and 10 (20%) as symmetric. There were no significant differences in demographic features at baseline between groups. There was, however, a difference in the time between the first and last visit and in Braak stage. Although not statistically different, 54% of the participants with right-dominant PPAOS were found to have corticobasal degeneration (CBD) pathology at autopsy, while 73% of those with left-dominant PPAOS were found to have progressive supranuclear palsy (PSP) pathology.
Table 1:
Demographic, genetic and pathologic features
| Variable of interest | Left-dominant (N=25) | Right-dominant (N=16) | Symmetric (N=10) | p-value |
|---|---|---|---|---|
| Men % | 12 (48%) | 7 (44%) | 4 (40%) | 0.93 |
| Education (yrs.) | 16.0 (15.0, 18.0) | 16.0 (15.0, 18.0) | 16.5 (13.0, 18.0) | 0.88 |
| Right Handedness % | 21 (84%) | 12 (75%) | 10 (100%) | 0.25 |
| Family history present % | 4 (21.1%) | 6 (50%) | 1 (17%) | 0.24 |
| Race % (Caucasian) | 25 (100%) | 15 (94%) | 10 (100%) | 0.51 |
| Age at onset (yrs.) | 69.6 (62.7, 74.1) | 65.6 (57.2, 72.2) | 67.0 (56.1, 74.7) | 0.52 |
| Age at baseline FDG-PET (yrs.) | 73.6 (65.2, 78.3) | 69.7 (59.8, 74.4) | 70.5 (59.1, 78.6) | 0.50 |
| Time from onset to baseline (yrs.) | 3.6 (2.2, 4.5) | 3.1 (2.0, 5.8) | 3.4 (2.6, 4.1) | 0.98 |
| Number with longitudinal visits | 18 (72%) | 14 (88%) | 6 (60%) | 0.28 |
| Time between baseline and last clinical visit, yrs. | 4.6 (2.6, 5.8) | 3.0 (1.9, 3.1) | 2.3 (1.9, 3.7) | 0.04 |
| PiB Status % (pos = >1.48) | 8 (32%) | 4 (26.7%) | 0 (0%) | 0.16 |
| Positive APOE ε4 (%) | 8 (36%) | 3 (20%) | 0 (0%) | 0.104 |
| PATHOLOGY | ||||
| N=11 | N=11 | N=1 | ||
| Age at death (yrs.) | 78.0 (67.75, 83.0) | 73.5 (64.25, 82.75) | 59 | 0.23 |
| PSP/CBD (%) | *8/2 (73%/18%) | 5/6 (46%/54%) | 0/1/ (0%/100%) | 0.12 |
| Braak Stage | 3 (3, 4) | 2.5 (2, 3) | 4 | 0.03 |
| Thal Phase | 2 (1,2) | 0 (0, 3) | 0 | 0.29 |
| FDG-PET SUVRs | ||||
| Left SMA | 1.41 (1.32, 1.54) | 1.44 (1.35, 1.51) | 1.45 (1.32, 1.52) | 0.93 |
| Right SMA | 1.47 (1.38, 1.58) | 1.32 (1.27, 1.41) | 1.46 (1.30, 1.51) | 0.003 |
| Left Broca’s | 1.48 (1.38, 1.57) | 1.53 (1.43, 1.64) | 1.47 (1.42, 1.62) | 0.37 |
| Right Broca’s | 1.60 (1.52, 1.64) | 1.55 (1.42, 1.60) | 1.55 (1.45, 1.67) | 0.29 |
Data are presented as median and interquartile range, unless otherwise indicated. Abbreviations: APOE, apolipoprotein epsilon; CBD, corticobasal degeneration; FDG, [18F]- fluorodeoxyglucose; PET, positron emission tomography; PiB, Pittsburgh Compound B; PSP, progressive supranuclear palsy; SMA, supplementary motor area; SUVR, standard uptake value ratio.
One additional patient did not have PSP or CBD but instead had FTLD-TDP
Figure 2:
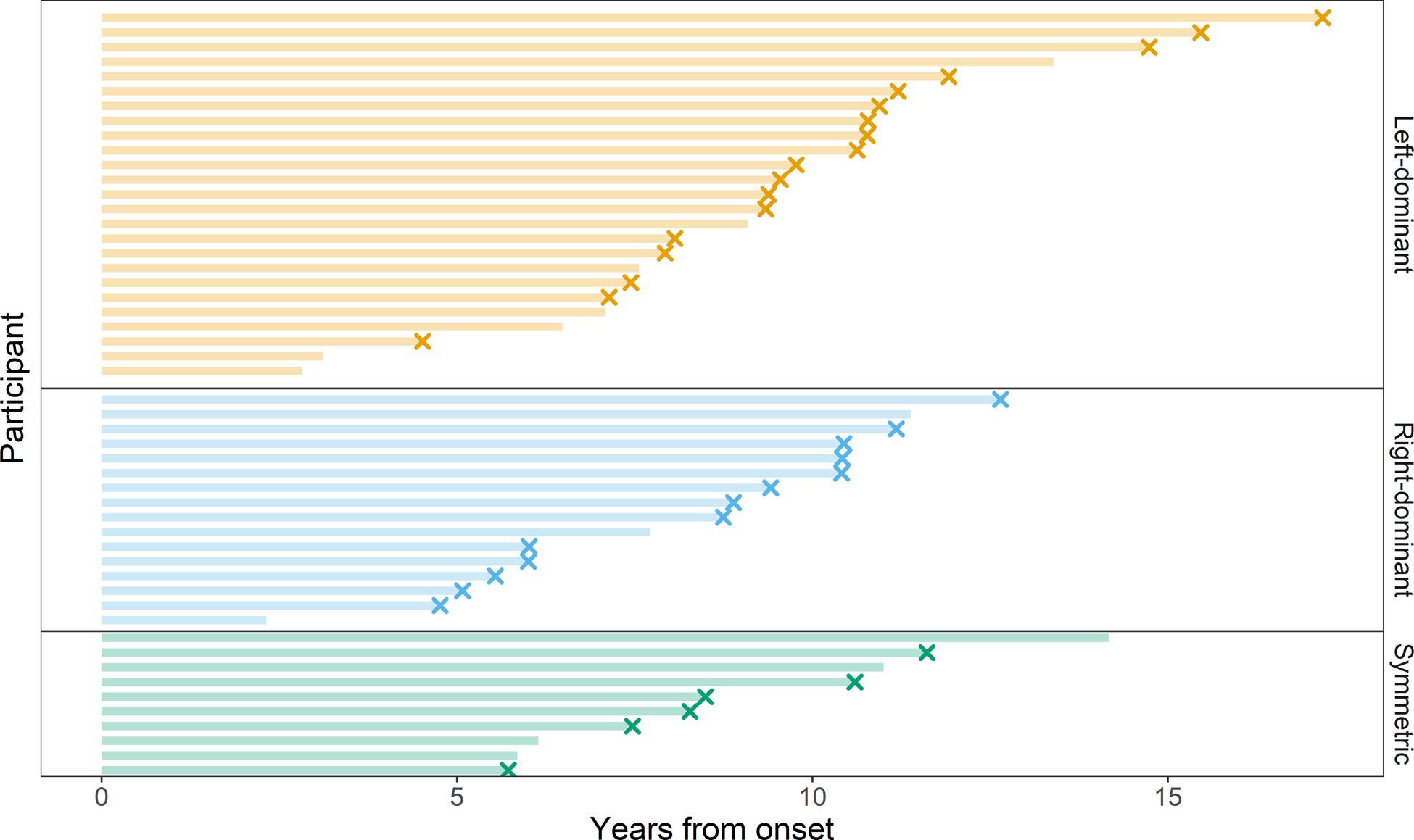
Swim plot demonstrating date of onset to date of death. Each participant is represented by a horizontal segment in this plot, colored, and arranged by left-dominant, right-dominant, and symmetric categorization groups. The horizontal bars represent the time from disease onset to death. An “X” at the end of the segment indicates that the patient has died.
Cross-sectional and longitudinal clinical associations
The groups showed no significant clinical differences at baseline (Table 2). Longitudinally, differences across the three groups were observed in the rate of worsening over time in Parkinsonism, ideomotor apraxia, and behavioral disturbances, including disinhibition and negative behaviors subscores, with faster rates of worsening for right-predominant PPAOS (Table 3). Differences across groups were also observed in the rate of worsening over time in dysarthria with faster rates of worsening in symmetric PPAOS compared to left-dominant PPAOS. All findings survived correction for multiple comparisons, except the difference in Parkinsonism.
Table 2:
Clinical features at baseline
| Clinical scale/diagnoses | Left-dominant (N=25) | Right-dominant (N=16) | Symmetric (N=10) | p-value |
|---|---|---|---|---|
| MoCA /30 | 28.0 (26.5, 29.0) | 26.5 (24.0, 29.0) | 28.0 (26.5, 29.0) | 0.76 |
| UPDRS III / 180 | 9.0 (5.0, 14.5) | 11.5 (4.0, 23.0) | 10.5 (5.0, 22.3) | 0.83 |
| NPI-Q /36 | 1.0 (0, 3.0) | 2.5 (1.0, 4.8) | 1.0 (0.0, 2.3) | 0.12 |
| Total FBI /72 | 8.0 (3.0, 17.5) | 6.0 (4.0, 11.5) | 7.5 (3.8, 11.3) | 0.89 |
| Disinhibition /36 | 2.5 (0, 5.0) | 1.0 (1.00, 2.0) | 2.0 (0.3, 3.0) | 0.66 |
| Negative behaviors /36 | 5.5 (3.0, 11.3) | 4.0 (3.0, 10.0) | 6.0 (3.25, 8.3) | 0.91 |
| AOS Subtype: (Phonetic/Prosodic/mixed) | 9/15/1(36%/60%/4%) | 8/6/2(50%/38%/12%) | 3/7/0(30%/70%/0%) | 0.25 |
| Number (%) with dysarthria | 6 (24%) | 4 (25%) | 4 (40%) | 0.61 |
| WAB-Praxis /60 | 58.0 (57.0, 60.0) | 58.5 (57.0, 59.0) | 58.0 (57.8, 59.0) | 0.99 |
| WAB-AQ /100 | 97.6 (96.9, 98.8) | 96.9 (95.3, 99.4) | 98.6 (96.4, 99.5) | 0.58 |
| NAT/10 | 9.0 (9.0, 10.0) | 10.0 (9.0, 10.0) | 10.0 (8.8, 10.0) | 0.45 |
| ASRS total score /52 | 15.0 (10.5, 19.5) | 14.0 (11.0, 27.3) | 17.0 (13.5, 23.5) | 0.52 |
| Dysarthria severity /4 | 1.0 (1.0, 1.9) | 1.8 (1.1, 2.8) | 1.0 (1.0, 1.4) | 0.31 |
| MSD /10 | 7.0 (6.0, 8.0) | 7.0 (5.3, 8.0) | 6.5 (5.9, 8.0) | 0.91 |
Data shown as median and interquartile range
Abbreviations: Western Aphasia Battery-Aphasia Quotient (WAB-AQ), Western Aphasia Battery-Praxis (WAB-Praxis), Northwestern Anagram Test (NAT), Apraxia of Speech Rating Scale (ASRS), Unified Parkinson’s Disease Rating Scale III (UPDRS III), Montreal Cognitive Assessment (MoCA); Motor Speech Disorder severity (MSD), Neuropsychiatric Inventory Questionnaire (NPI-Q), Frontal Behavior Inventory (FBI).
Table 3:
Annual rates of change in clinical measures
| Clinical Scale | Left-dominant (N=18) | Right-dominant (N=14) | Symmetric (N=6) | P-value |
|---|---|---|---|---|
| MoCA /30 | 2.0 (0.7, 2.9) | 3.5 (1.7, 7.7) | 3.3 (0.0, 7.1) | 0.25 |
| UPDRS III /180 | 8.6 (4.0, 11.0) | 14.7 (8.4, 26.5) | 14.7 (9.0, 18.9) | 0.01 |
| NPI-Q /36 | 0.3 (−0.1, 1.0) | 1.25 (0.8, 3.8) | 0 (−0.3, 3.0) | 0.11 |
| FBI total/72 | 2.0 (0.7, 3.8) | 8.0 (4.0, 10.34) | 3.25 (0.4, 11.8) | 0.009 a |
| FBI - Negative behaviors /36 | 1.5 (0.5, 2.9) | 5.0 (2.2, 7.0) | 3.25 (1.1, 7.3) | 0.01 a |
| FBI - Disinhibition /36 | 0.2 (0.0, 0.7) | 2.0 (0.8, 5.0) | 0.0 (−0.8, 4.50) | 0.02 a |
| WAB-Praxis /60 * | 1.8 (1.3, 4.5) | 6.33 (4.6, 7.0) | 4.90 (1.75, 15.25) | 0.02 a |
| WAB-AQ /100 * | 1.43 (0.4, 4.6) | 2.1 (1.1, 5.7) | 1.40 (0.4, 6.25) | 0.28 |
| NAT /10 * | 0 (−0.3, 1.7) | 1.0 (0.3, 2.0) | 0.0 (0.0, 2.0) | 0.16 |
| ASRS total score /52 | 4.3 (3.1, 7.2) | 5.1 (3.1, 6.3) | 4.8 (1.8, 9.6) | >0.99 |
| Dysarthria severity /4 | 0.1 (0.0, 0.5) | 0.3 (0.2, 0.6) | 1.0 (0.4, 1.2) | 0.05 b |
| MSD /10 * | 0.8 (0.5, 1.0) | 1.0 (0.7, 2.0) | 1.50 (0.9, 2.1) | 0.09 |
Data shown as median and interquartile range. Clinical scales shown as points change per year.
Abbreviations: Western Aphasia Battery-Aphasia Quotient (WAB-AQ), Western Aphasia Battery-Praxis (WAB-Praxis), Northwestern Anagram Test (NAT), Apraxia of Speech Rating Scale (ASRS), Unified Parkinson’s Disease Rating Scale III (UPDRS III), Montreal Cognitive Assessment (MoCA) test, Motor Speech Disorder severity (MSD), Neuropsychiatric Inventory Questionnaire (NPI-Q), Frontal Behavior Inventory (FBI).
Higher rates reflect greater decrease in raw scores
Significant difference between left-dominant and right-dominant PPAOS using Dunn’s multiple comparison test
Significant difference between left-dominant and symmetric PPAOS using Dunn’s multiple comparison test
The rate of worsening in behavioral disturbances, including negative behaviors and disinhibition, were very good (AUROC≥0.80) at distinguishing right-dominant and left-dominant PPAOS (Table 4). Rates of worsening in Parkinsonism and ideomotor apraxia provided acceptable (AUROC 0.75–0.79) discrimination between right-dominant and left-dominant PPAOS. Rates of worsening in Parkinsonism and dysarthria were very good at discriminating between left-dominant and symmetric PPAOS, while rates of worsening of negative behaviors and ideomotor apraxia provided acceptable discrimination. Rates of worsening in dysarthria provided acceptable discrimination between right-dominant and symmetric PPAOS.
Table 4:
AUROC comparing groups using annual rate of change in clinical measures.
| Left-dominant vs Right-dominant | Left-dominant vs Symmetric | Right-dominant vs Symmetric | |
|---|---|---|---|
| UPDRS III | 0.75 (0.57 to 0.93) | 0.82 (0.59 to 1.0) | 0.51 (0.24 to 0.78) |
| FBI | 0.84 (0.67 to 1.0) | 0.65 (0.28 to 1.0) | 0.62 (0.28 to 1.0) |
| Negative behaviors | 0.81 (0.64 to 0.99) | 0.73 (0.43 to 1.0) | 0.55 (0.21 to 0.89) |
| Disinhibition | 0.82 (0.65 to 0.99) | 0.57 (0.19 to 0.96) | 0.63 (0.26 to 0.99) |
| Dysarthria severity | 0.63 (0.37 to 0.91) | 0.89 (0.70 to 1.0) | 0.79 (0.47 to 1.0) |
| WAB Praxis | 0.79 (0.62 to 0.96) | 0.71 (0.48 to 0.94) | 0.57 (0.25 to 0.89) |
| WAB-AQ | 0.66 (0.47 to 0.85) | 0.62 (0.35 to 0.89) | 0.57 (0.24 to 0.89) |
| MoCA | 0.69 (0.48 to 0.89) | 0.60 (0.26 to 0.94) | 0.53 (0.22 to 0.83) |
| NPI-Q | 0.74 (0.55 to 0.94) | 0.58 (0.29 to 0.88) | 0.58 (0.28 to 0.88) |
| NAT | 0.70 (0.51 to 0.89) | 0.58 (0.35 to 0.82) | 0.68 (0.37 to 0.99) |
| ASRS | 0.51 (0.30 to 0.73) | 0.51 (0.19 to 0.83) | 0.54 (0.20 to 0.89) |
| MSD | 0.70 (0.51 to 0.89) | 0.72 (0.50 to 0.95) | 0.53 (0.25 to 0.81) |
Data shown as area and 95% confidence interval. Abbreviations: Unified Parkinson’s Disease Rating Scale III (UPDRS III), Western Aphasia Battery-Praxis (WAB-Praxis), Western Aphasia Battery-Aphasia Quotient (WAB-AQ), Frontal Behavioral Inventory (FBI), Neuropsychiatric Inventory Questionnaire (NPI-Q), Northwestern Anagram Test (NAT), Motor Speech Disorder severity (MSD), Apraxia of Speech Rating Scale (ASRS).
SPM analysis
In the voxel-level analysis (Figure 3), left-dominant PPAOS showed hypometabolism in the left SMA, middle-frontal LPC, motor cortex, and posterior Broca’s area, compared to the healthy controls, with subtle involvement of the right SMA. Right-dominant PPAOS showed hypometabolism in the right SMA, LPC, and motor cortices compared to controls, with subtle involvement of the left SMA. Symmetric PPAOS showed hypometabolism in the left and right motor cortex and left SMA compared to controls.
Figure 3:
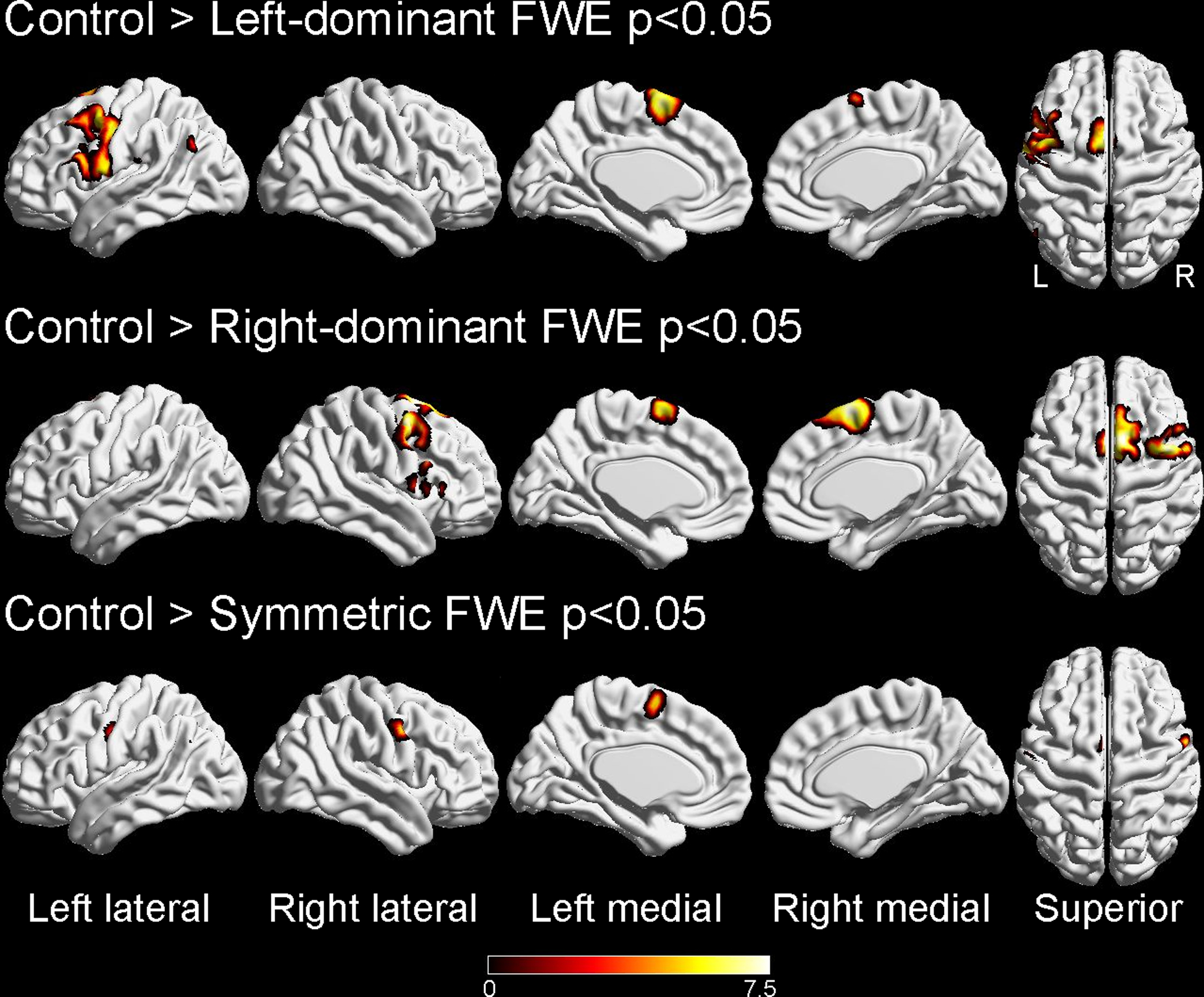
Voxel-level analysis of left-dominant, right-dominant, and symmetric primary progressive apraxia of speech (PPAOS) groups compared to healthy controls. Results are shown on three-dimensional brain renderings at a statistical threshold of p<0.05 after correction for multiple comparisons using family-wise error correction. Renders were generated using the BrainNet Viewer (http://www.nitrc.org/projects/bnv/).
Semi-quantitative and quantitative regional analyses
All PPAOS participants showed some degree of hypometabolism in the LPC/SMA on visual assessment which on average was rated as mild for all three groups. The degree of hypometabolism in Broca’s area was minimal on average for all three groups and was always less in severity than in the LPC/SMA, which was also observed in the quantitative FDG-PET SUVR values (Table 1). Of the 51 PPAOS participants, 62% did not have any evidence of hypometabolism in Broca’s area. There were no right or left-dominant PPAOS participants with isolated hypometabolism in one hemisphere on visual inspection. That is, there was always at least minimal hypometabolism in the non-dominant hemisphere. As expected, we observed asymmetry in the SMA SUVRs in the left-predominant and right-predominant groups, and asymmetry in Broca’s area in the left-predominant group (Table 1).
DAT scan analysis
Of the 13 DAT scans (six left-dominant PPAOS, five right-dominant PPAOS, and two symmetric PPAOS), five (26%) were abnormal (two left-dominant PPAOS, two right-dominant PPAOS, and one symmetric PPAOS) (Figure 4). Both abnormal scans from the left-dominant group showed reduced binding in the left putamen. The two scans from the right-dominant group showed reduced binding in the right putamen in one and bilateral putamen and right caudate in the other. The DAT scan from the symmetric group showed bilateral reduced binding in the putamen, possibly slightly worse on the right.
Figure 4:
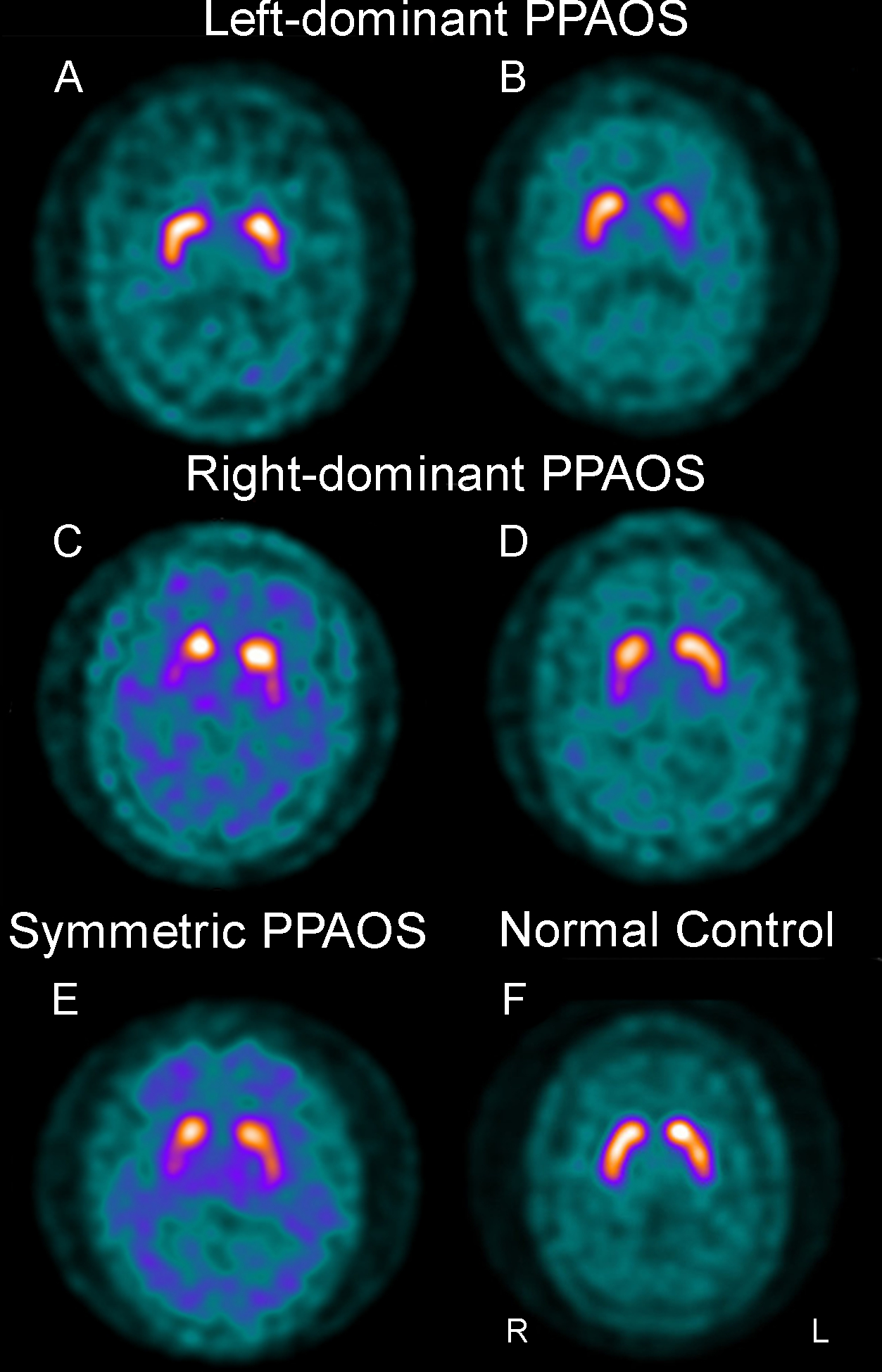
Dopamine active transfer (DAT) scans showing abnormal uptake in the striatum in five cases (A-E) compared to a normal scan (F). PPAOS, primary progressive apraxia of speech
DISCUSSION
In this study, we found that approximately half of the participants with PPAOS had a left-dominant pattern of LPC and SMA hypometabolism, with the other half split between right-dominant and symmetric. Longitudinal rates of clinical decline in behavior and motor function were faster in the right-dominant participants.
In this study of participants who present with an isolated motor speech disorder known as PPAOS, we show that such a presentation is not linked to any one specific hemisphere. This lack of hemispheric dominance in PPAOS is very different from what is observed in nfvPPA where damage is almost exclusively associated with isolated or dominant left hemisphere atrophy and hypometabolism. In fact, nfvPPA has very rarely been reported to be associated with right hemisphere dominant atrophy or hypometabolism [36–38]. To try to understand this asymmetry in PPAOS we assessed underlying molecular pathology. Nearly all of our PPAOS participants that came to autopsy had an underlying 4R tauopathy (96%), either CBD or PSP, consistent with previous studies that have associated AOS with 4R tauopathies [15, 17, 39]. Not surprisingly, we found CBD to be the underlying pathology in many of our cases as CBD is typically associated with asymmetric neurodegeneration and an asymmetric clinical presentation [40]. On-the-other hand, we also found PSP pathology. PSP pathology is typically associated with symmetric clinical and neuroimaging findings [41] and hence, the finding of PSP pathology in almost three-quarters of the left-dominant PPAOS participants is novel.
Hemispheric dominance did not appear to impact clinical features at baseline. However, significant differences emerged with disease progression. Most notably, right-dominant PPAOS was associated with faster worsening of parkinsonism, ideomotor apraxia, and behavioral dysfunction, particularly compared to left-dominant PPAOS. In fact, rates of worsening in behavioral dyscontrol and ideomotor apraxia provided very good AUROC effect sizes to differentiate right-dominant and left-dominant PPAOS. The reason for the faster progression of these signs and symptoms in right-dominant PPAOS is not entirely clear, although we speculate that it may reflect the higher proportion of underlying CBD pathology in the right-dominant group given that CBD is associated with a shorter time from onset to death compared to PSP. The symmetric PPAOS group also showed faster rates of worsening in parkinsonism, ideomotor apraxia, and negative behaviors compared to the left-dominant group, perhaps suggesting that involvement of the right hemisphere, to an equal or greater degree, compared to the left hemisphere is what is playing a role in faster progression.
The symmetric PPAOS group showed the fastest rates of worsening of dysarthria and a trend for a faster decline in motor speech, as assessed by the MSD. We suspect that the trend with MSD is being driven by the worsening of dysarthria, rather than a worsening of AOS. It is unclear why the symmetric group would show the fastest decline in dysarthria, although it is not necessarily surprising given that hypometabolism has been reported to progressively evolve to affect the lateral motor cortex in PPAOS, a region that is associated with dysarthria [42, 43]. The fact that AOS severity did not differ at baseline or longitudinally across groups raises the possibility that the presence of AOS is not impacted by hemispheric asymmetry and that damage to either the left or right premotor cortex can result in AOS, perhaps through damage to structural and functional connections between and from these regions [14]. Indeed, PPAOS patients show striking degeneration of the callosal fibers that connect the left and right SMA [44]. It should be noted that all PPAOS participants showed hypometabolism in both hemispheres even if patterns were asymmetric (i.e., we did not find any patient with isolated left or right hypometabolism). Hence, it is also possible that damage to both the left and right premotor cortex (regardless of the degree of damage) is necessary to produce AOS although we cannot exclude the fact that AOS results from damage to one hemisphere which is consistently affected across groups. One could argue that if AOS was linked to just one hemisphere, it would more likely be the left hemisphere since clinically silent strokes are more common in the right hemisphere [45]. With that said we did not find AOS severity to be worse in the left-dominant patients. Finally, these possible scenarios would explain why the proportion of prosodic and phonetic PPAOS subtypes did not differ across the three groups.
Although we did not find a significant difference across the groups for the AOS subtype and for pathology, it should be noted that 60% of the left-dominant group had prosodic AOS and 73% had PSP pathology, while 50% of the right-dominant group had phonetic AOS and 54% had CBD pathology. This is in keeping with what we have previously shown that prosodic AOS is associated with PSP pathology while phonetic AOS is associated with CBD pathology [6]. A previous study found that abnormal speech timing, a type of dysprosody is associated with CBD pathology in patients with nvfPPA [46]. Those findings may appear contradictory; however, it is difficult to compare both studies since the referenced paper did not specify the breakdown of dysarthria and AOS subtype. In addition, our study excluded patients with aphasia and greater aphasia severity may have influenced the differences in results (e.g., linguistic interference inflating at least the pause duration measure). In our view, dysprosody is an umbrella term that may capture disruptions in speech rhythm that occur either secondary to dysarthria (neuromuscular execution difficulties) or AOS (motor planning or programming deficits).
Of the 13 PPAOS patients that completed a DAT scan five were abnormal suggesting that approximately a third of PPAOS patients already have dopamine deficiency at the time of presentation[47]. We did not find a difference in the frequency of abnormal DAT scans across groups possibly because of the limited sample size but it is equally likely that group designation is not associated with dopamine deficiency especially given that PSP and CBD pathologies are both associated with dopamine deficiency states and abnormal DAT scans [48]. Importantly, we did find hemispheric dominance of DAT scan uptake to mirror hemispheric designation of left- vs right vs symmetric based on FDG-PET. This independent DAT scan finding further validates the FDG-PET group designation. In fact, the VBM finding, another independent analysis also validates the group designation showing left-sided predominance in the left-dominant group, right-sided predominance in the right-dominant group, and symmetric hypometabolism in the symmetric group.
There are several strengths to our study including the sample size and that the patients were prospectively followed over several years. All patients were also well-characterized using a multidisciplinary standardized approach, by expert neurologists, speech-language pathologists, and neuropsychologist. Our statistical analysis corrected for multiple comparisons and the AUROCs provided information on strength of group discrimination, which is especially important given the limited power for three group comparisons. There were also some limitations to our study such as a limited number of cognitive tests to evaluate right hemisphere functioning and that not all patients completed a DAT scan or had an autopsy. We also acknowledge that the integral assessments included in this investigation were established based on perceptual judgments of motor speech output and visual assessment of FDG-PET neuroimaging findings. Lastly, we had a 24% attrition rate due to participants becoming too severe to continue to undergo further longitudinal testing or simply being unable to follow up for future testing.
Acknowledgment:
Funding
This project is funded by NIH grants R01 DC14942 and R01 DC12519.
Footnotes
Competing Interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethics Approval
This study was approved by the Mayo Clinic Institutional Review Board (IRB numbers 09– 008772, 12–008988 and 17–002468) and all patients, or their proxies signed a written informed consent form before taking part in any research activities in accordance with the Declaration of Helsinki.
Data Availability
Anonymized data are available from the corresponding author upon request from any qualified investigator for purposes of replicating procedures and results.
References:
- [1].Botha H, Josephs KA. Primary Progressive Aphasias and Apraxia of Speech. Continuum (Minneap Minn). 2019. 25: 101–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012. 135: 1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Duffy JR, Josephs KA. The diagnosis and understanding of apraxia of speech: why including neurodegenerative etiologies may be important. J Speech Lang Hear Res. 2012. 55: S1518–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Utianski RL, Duffy JR, Clark HM, et al. Clinical Progression in Four Cases of Primary Progressive Apraxia of Speech. Am J Speech Lang Pathol. 2018. 27: 1303–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Utianski RL, Clark HM, Duffy JR, Botha H, Whitwell JL, Josephs KA. Communication Limitations in Patients With Progressive Apraxia of Speech and Aphasia. Am J Speech Lang Pathol. 2020. 29: 1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Utianski RL, Duffy JR, Clark HM, et al. Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain Lang. 2018. 184: 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Duffy JR, Utianski RL, Josephs KA. Primary Progressive Apraxia of Speech: From Recognition to Diagnosis and Care. Aphasiology. 2021. 35: 560–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Josephs KA, Duffy JR, Strand EA, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013. 81: 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982. 11: 592–598. [DOI] [PubMed] [Google Scholar]
- [10].Mesulam MM. Primary progressive aphasia--a language-based dementia. N Engl J Med. 2003. 349: 1535–1542. [DOI] [PubMed] [Google Scholar]
- [11].Duffy JR, Strand EA, Clark H, Machulda M, Whitwell JL, Josephs KA. Primary progressive apraxia of speech: clinical features and acoustic and neurologic correlates. Am J Speech Lang Pathol. 2015. 24: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Josephs KA, Duffy JR. Apraxia of speech and nonfluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Curr Opin Neurol. 2008. 21: 688–692. [DOI] [PubMed] [Google Scholar]
- [13].Tetzloff KA, Duffy JR, Strand EA, et al. Clinical and imaging progression over 10 years in a patient with primary progressive apraxia of speech and autopsy-confirmed corticobasal degeneration. Neurocase. 2018. 24: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Botha H, Utianski RL, Whitwell JL, et al. Disrupted functional connectivity in primary progressive apraxia of speech. Neuroimage Clin. 2018. 18: 617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006. 129: 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Josephs KA, Duffy JR, Strand EA, et al. The evolution of primary progressive apraxia of speech. Brain. 2014. 137: 2783–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Josephs KA, Duffy JR, Clark HM, et al. A molecular pathology, neurobiology, biochemical, genetic and neuroimaging study of progressive apraxia of speech. Nat Commun. 2021. 12: 3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kertesz A Western Aphasia Battery (Revised). San Antonio, Tx: PsychCorp, 2007. [Google Scholar]
- [19].Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2009. 24: 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Strand EA, Duffy JR, Clark HM, Josephs K. The apraxia of speech rating scale: a tool for diagnosis and description of apraxia of speech. J Commun Disord. 2014. 51: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008. 23: 2129–2170. [DOI] [PubMed] [Google Scholar]
- [22].Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005. 53: 695–699. [DOI] [PubMed] [Google Scholar]
- [23].Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders : official journal of the Movement Disorder Society. 2008. 23: 2129–2170. [DOI] [PubMed] [Google Scholar]
- [24].Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. The Journal of neuropsychiatry and clinical neurosciences. 2000. 12: 233–239. [DOI] [PubMed] [Google Scholar]
- [25].Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci. 1997. 24: 29–36. [DOI] [PubMed] [Google Scholar]
- [26].Josephs KA, Duffy JR, Strand EA, et al. APOE ε4 influences β-amyloid deposition in primary progressive aphasia and speech apraxia. Alzheimers Dement. 2014. 10: 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Whitwell JL, Tosakulwong N, Weigand SD, et al. Longitudinal Amyloid-β PET in Atypical Alzheimer’s Disease and Frontotemporal Lobar Degeneration. J Alzheimers Dis. 2020. 74: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995. 36: 1238–1248. [PubMed] [Google Scholar]
- [29].Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991. 41: 479–486. [DOI] [PubMed] [Google Scholar]
- [30].Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991. 82: 239–259. [DOI] [PubMed] [Google Scholar]
- [31].Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002. 58: 1791–1800. [DOI] [PubMed] [Google Scholar]
- [32].Dickson DW. Neuropathology of Pick’s disease. Neurology. 2001. 56: S16–20. [DOI] [PubMed] [Google Scholar]
- [33].Dickson DW, Bergeron C, Chin SS, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002. 61: 935–946. [DOI] [PubMed] [Google Scholar]
- [34].Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci. 2011. 45: 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology. 1994. 44: 2015–2019. [DOI] [PubMed] [Google Scholar]
- [36].Jeong EH, Lee YJ, Kwon M, Kim JS, Na DL, Lee JH. Agrammatic primary progressive aphasia in two dextral patients with right hemispheric involvement. Neurocase. 2014. 20: 46–52. [DOI] [PubMed] [Google Scholar]
- [37].Repetto C, Manenti R, Cotelli M, et al. Right hemisphere involvement in non-fluent primary progressive aphasia. Behav Neurol. 2007. 18: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Spinelli EG, Caso F, Agosta F, et al. A multimodal neuroimaging study of a case of crossed nonfluent/agrammatic primary progressive aphasia. J Neurol. 2015. 262: 2336–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Deramecourt V, Lebert F, Debachy B, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010. 74: 42–49. [DOI] [PubMed] [Google Scholar]
- [40].Josephs KA, Tang-Wai DF, Edland SD, et al. Correlation between antemortem magnetic resonance imaging findings and pathologically confirmed corticobasal degeneration. Arch Neurol. 2004. 61: 1881–1884. [DOI] [PubMed] [Google Scholar]
- [41].Stamelou M, Knake S, Oertel WH, Hoglinger GU. Magnetic resonance imaging in progressive supranuclear palsy. J Neurol. 2011. 258: 549–558. [DOI] [PubMed] [Google Scholar]
- [42].Clark HM, Duffy JR, Whitwell JL, Ahlskog JE, Sorenson EJ, Josephs KA. Clinical and imaging characterization of progressive spastic dysarthria. Eur J Neurol. 2014. 21: 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kumral E, Celebisoy M, Celebisoy N, Canbaz DH, Calli C. Dysarthria due to supratentorial and infratentorial ischemic stroke: a diffusion-weighted imaging study. Cerebrovasc Dis. 2007. 23: 331–338. [DOI] [PubMed] [Google Scholar]
- [44].Valls Carbo A, Reid RI, Tosakulwong N, et al. Tractography of supplementary motor area projections in progressive speech apraxia and aphasia. Neuroimage Clin. 2022. 34: 102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Raghavan S, Graff-Radford J, Scharf E, et al. Study of Symptomatic vs. Silent Brain Infarctions on MRI in Elderly Subjects. Front Neurol. 2021. 12: 615024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].García AM, Welch AE, Mandelli ML, et al. Automated Detection of Speech Timing Alterations in Autopsy-Confirmed Nonfluent/Agrammatic Variant Primary Progressive Aphasia. Neurology. 2022. 99: e500–e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Seckin ZI, Whitwell JL, Utianski RL, et al. Ioflupane 123I (DAT scan) SPECT identifies dopamine receptor dysfunction early in the disease course in progressive apraxia of speech. J Neurol. 2020. 267: 2603–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kaasinen V, Kankare T, Joutsa J, Vahlberg T. Presynaptic Striatal Dopaminergic Function in Atypical Parkinsonism: A Metaanalysis of Imaging Studies. J Nucl Med. 2019. 60: 1757–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data are available from the corresponding author upon request from any qualified investigator for purposes of replicating procedures and results.


