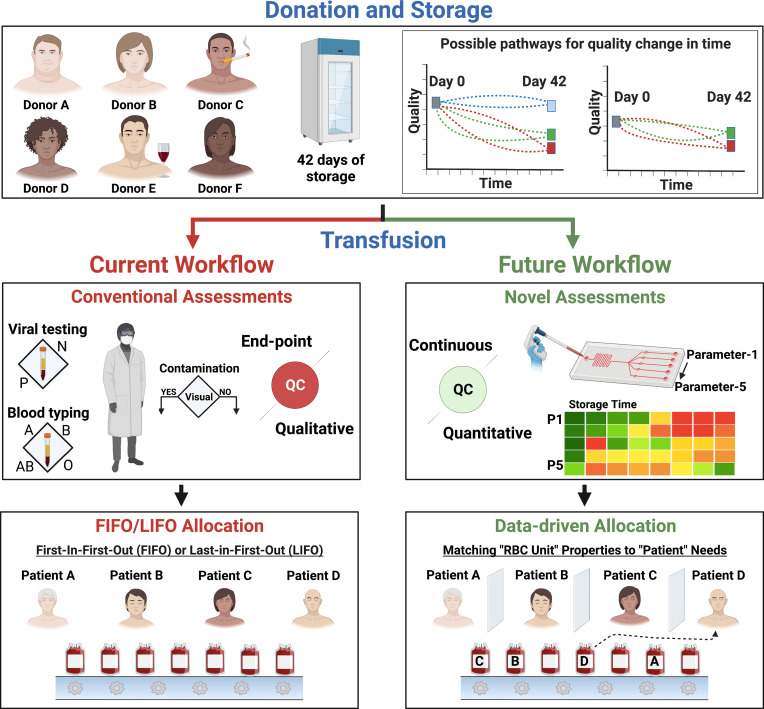
Fig. 1.
The current and proposed future workflow for RBC storage and transfusion medicine. Donors vary in sex, age, ethnicity, genetic disposition, body mass index, metabolism, and habits. These variations can lead to different initial quality levels for donated RBCs and to different quality changes during storage. Currently, blood banks store donated RBCs at 4 °C for 42 d. This storage can induce injuries collectively termed the “storage lesions” in a donor-, time-, and processing-dependent manner. Thus, the injury to each stored RBC unit can be unique. The current quality assessments performed on individual RBC units before transfusion are infectious disease marker testing and donor screening, blood typing, and visual inspection for bacterial contamination. These assessments are agreed upon and mandated by health agencies worldwide—including the FDA and CDC. RBC units are generally allocated following the “first-in-first-out” protocol. However, this workflow may result in posttransfusion complications and significantly reduced efficacy. The advances in LOC technologies armed with information from -omics and ML studies offer a better alternative. We envision a future where each RBC unit is assessed continuously during storage using multiple quality metrics based on objective unbiased measurements via LOC technologies. Patients with different medical conditions will then be administered the right RBC unit via data-driven allocation. Such an approach will maximize the efficacy of transfusion therapies and reduce complications.