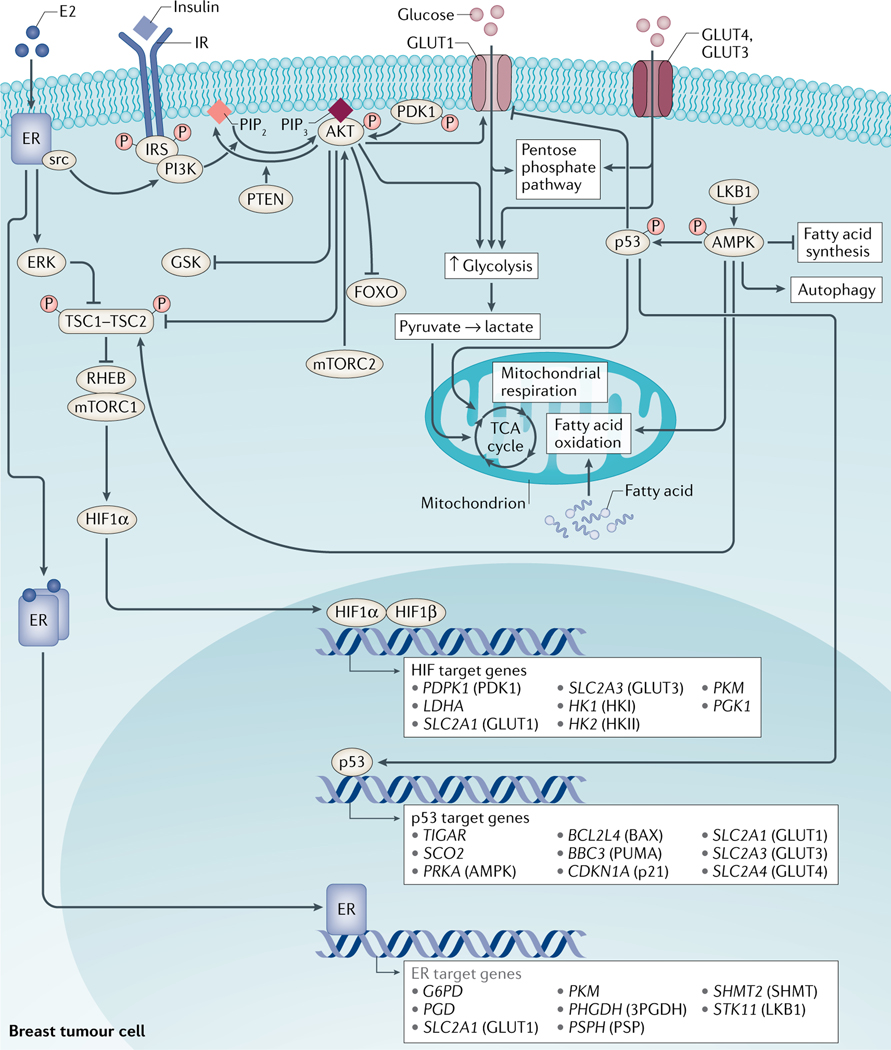
Fig. 2 |. Key metabolic pathways in breast cancer.
Several proteins orchestrate the regulation of energy metabolism in proliferating breast cancer cells and cells of the tumour microenvironment. Shifts in the mode of energy production from oxidative phosphorylation to aerobic glycolysis are tightly regulated by a number of proteins, including phosphoinositide 3-kinase (PI3K), RAC serine/threonine-protein kinase (AKT), mammalian target of rapamycin (mTOR) complex 1 (mTORC1), hypoxia-inducible factor 1α (HIF1α), liver kinase B1 (LKB1), AMP-activated protein kinase (AMPK) and p53. Activation of PI3K leads to the production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which binds to and activates AKT. In turn, AKT, via effects on tuberous sclerosis complex 2 (TSC2), activates mTORC1, which leads to the increased translation of several proteins, including HIF1α. AKT and HIF1α stimulate aerobic glycolysis by regulating glucose uptake and glycolytic enzymes. Conversely, binding of AMP to AMPK results in the phosphorylation of AMPK by LKB1 and in the regulation of downstream targets, including the inhibition of fatty acid synthesis, stimulation of fatty acid oxidation and autophagy. AMPK can also phosphorylate (and thereby stabilize) p53. In turn, p53 has been implicated in the downregulation of GLUT1 expression, inhibition of glycolysis and stimulation of oxidative phosphorylation.