Scheme 3.
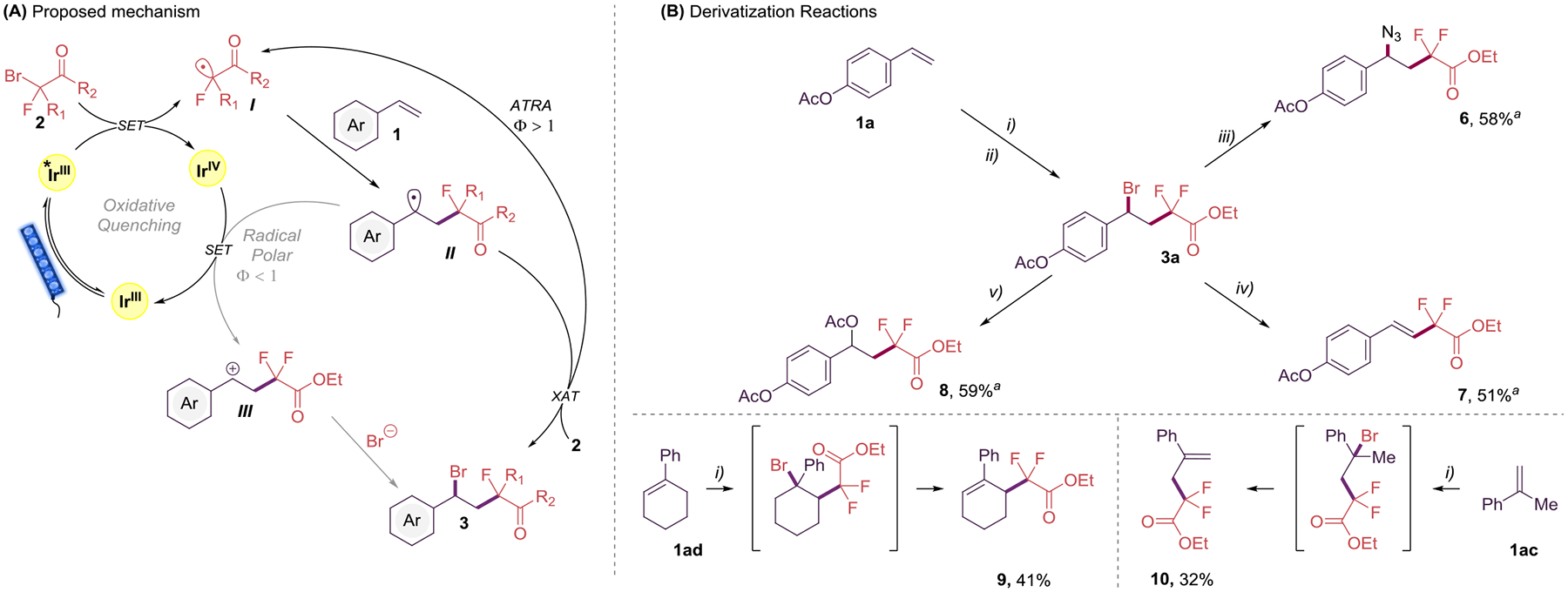
Proposed mechanism (A) and Derivatization Reactions (B)
(A) Proposed mechanism for the preparation of fluorinated benzyl bromides 3. (B) Derivatization reactions. Reaction conditions: i) 1 (0.5 mmol), 2a (1.0 mmol), Ir(ppy)3 (0.1 mol %) in MeCN (5.0 mL, 0.1 M), 24 h irradiation with blue LED strips (λmax = 456 nm). ii) Filtration through a short pad of silica. iii) NaN3 (0.75 mmol) in DMSO (5 mL, 0.1 M), overnight at rt. iv) EtONa (2.5 mmol) in EtOH (5 mL, 0.1 M), overnight at rt. v) AgOAc (0.5 mmol) in MeCN, 4 h at 50 °C. aIsolated yield from 1a.
